Back


Poster Session D - Tuesday Morning
Category: Functional Bowel Disease
D0259 - Efficacy of Rifaximin in Patients With Abdominal Bloating or Distension: A Systematic Review and Meta-Analysis
Tuesday, October 25, 2022
10:00 AM – 12:00 PM ET
Location: Crown Ballroom

Has Audio
.jpg)
Karan Sachdeva, MBBS
All India Institute of Medical Sciences
Delhi, Delhi, India
Presenting Author(s)
Umang Arora, MBBS, MD1, Karan Sachdeva, MBBS1, Prerna Garg, MBBS, MD1, Upendra Baitha, MBBS, MD1, Saurabh Kedia, MBBS, MD, DM1, M Kalaivani, PhD1, Vineet Ahuja, MBBS, MD, DM2, Arvind Kumar, MBBS, MD1, Piyush Ranjan, MBBS, MD1, Naval K. Vikram, MBBS, MD1, Sanjeev Sinha, MBBS, MD1, Ashutosh Biswas, MBBS, MD1, Naveet Wig, MBBS, MD1
1All India Institute of Medical Sciences, Delhi, Delhi, India; 2All India Institute of Medical Sciences, New Delhi, Delhi, India
Introduction: Abdominal bloating is a common complaint in patients with functional and organic bowel disease. Rifaximin, a non-absorbable antibiotic, has been tried for the treatment of this disease. We performed a systematic review and meta-analyses to study the efficacy of rifaximin in abdominal bloating and distension in patients with functional gastro-intestinal disorders (FGID).
Methods: We accessed four database (MEDLINE, Embase, SCOPUS, and Web of Science) to identify randomized placebo-controlled trials that utilized rifaximin in FGID. We excluded observational studies, studies including patients with organic bowel disorders such as inflammatory bowel disease, or when rifaximin was given for another indication such as HE. The protocol was registered on PROSPERO.
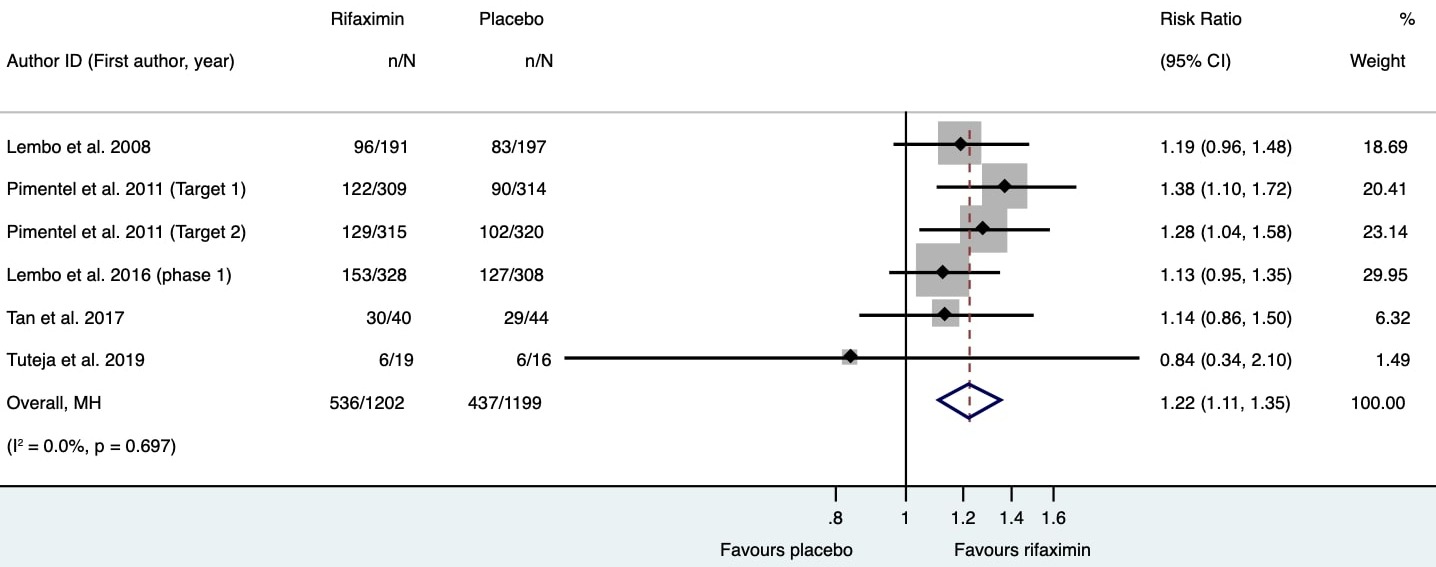
Results: 1426 articles were available of which 813 articles were screened after removing duplicates, and 34 articles were selected for full-text review. Finally, ten trials (3326 patients) were included. Rifaximin was administered in doses ranging from 400 to 1650 mg per day for one to two weeks. Rifaximin therapy led to higher likelihood of improvement in symptoms of bloating (44.6% vs 34.6%, RR 1.22, 95% CI 1.11, 1.35; n=2401 patients) without significant heterogeneity [Figure 1]. However, daily doses less than 1200 mg/day were similar to placebo (p=0.09). Bloating was quantified subjectively in 7 studies, and rifaximin led to greater reduction in bloating scores compared to placebo (standardized mean difference-0.3, 95% CI -0.51, -0.1, p=0.04) but carried significant heterogeneity (I2 =61.6%, p= 0.01). There is paucity of data on the role of repeat treatment with rifaximin among patients who developed recurrence of symptoms following a successful therapeutic trial. The trials included in the meta-anlaysis suffered from low risk of bias.
Discussion: Rifaximin therapy at doses of 1650 mg/day for 2 weeks led to an increased likelihood of improvement in bloating and distension as well as reducing subjective severity of these symptoms in patients with FGID.
Disclosures:
Umang Arora, MBBS, MD1, Karan Sachdeva, MBBS1, Prerna Garg, MBBS, MD1, Upendra Baitha, MBBS, MD1, Saurabh Kedia, MBBS, MD, DM1, M Kalaivani, PhD1, Vineet Ahuja, MBBS, MD, DM2, Arvind Kumar, MBBS, MD1, Piyush Ranjan, MBBS, MD1, Naval K. Vikram, MBBS, MD1, Sanjeev Sinha, MBBS, MD1, Ashutosh Biswas, MBBS, MD1, Naveet Wig, MBBS, MD1. D0259 - Efficacy of Rifaximin in Patients With Abdominal Bloating or Distension: A Systematic Review and Meta-Analysis, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1All India Institute of Medical Sciences, Delhi, Delhi, India; 2All India Institute of Medical Sciences, New Delhi, Delhi, India
Introduction: Abdominal bloating is a common complaint in patients with functional and organic bowel disease. Rifaximin, a non-absorbable antibiotic, has been tried for the treatment of this disease. We performed a systematic review and meta-analyses to study the efficacy of rifaximin in abdominal bloating and distension in patients with functional gastro-intestinal disorders (FGID).
Methods: We accessed four database (MEDLINE, Embase, SCOPUS, and Web of Science) to identify randomized placebo-controlled trials that utilized rifaximin in FGID. We excluded observational studies, studies including patients with organic bowel disorders such as inflammatory bowel disease, or when rifaximin was given for another indication such as HE. The protocol was registered on PROSPERO.
Results: 1426 articles were available of which 813 articles were screened after removing duplicates, and 34 articles were selected for full-text review. Finally, ten trials (3326 patients) were included. Rifaximin was administered in doses ranging from 400 to 1650 mg per day for one to two weeks. Rifaximin therapy led to higher likelihood of improvement in symptoms of bloating (44.6% vs 34.6%, RR 1.22, 95% CI 1.11, 1.35; n=2401 patients) without significant heterogeneity [Figure 1]. However, daily doses less than 1200 mg/day were similar to placebo (p=0.09). Bloating was quantified subjectively in 7 studies, and rifaximin led to greater reduction in bloating scores compared to placebo (standardized mean difference-0.3, 95% CI -0.51, -0.1, p=0.04) but carried significant heterogeneity (I2 =61.6%, p= 0.01). There is paucity of data on the role of repeat treatment with rifaximin among patients who developed recurrence of symptoms following a successful therapeutic trial. The trials included in the meta-anlaysis suffered from low risk of bias.
Discussion: Rifaximin therapy at doses of 1650 mg/day for 2 weeks led to an increased likelihood of improvement in bloating and distension as well as reducing subjective severity of these symptoms in patients with FGID.

Figure: Forest plot depicting the proportion of patients demonstrating improvement in abdominal bloating or distension in each study. The pooled risk ratio (1.22, 95% CI 1.11, 1.35) favors intervention (rifaximin).
Disclosures:
Umang Arora indicated no relevant financial relationships.
Karan Sachdeva indicated no relevant financial relationships.
Prerna Garg indicated no relevant financial relationships.
Upendra Baitha indicated no relevant financial relationships.
Saurabh Kedia indicated no relevant financial relationships.
M Kalaivani indicated no relevant financial relationships.
Vineet Ahuja indicated no relevant financial relationships.
Arvind Kumar indicated no relevant financial relationships.
Piyush Ranjan indicated no relevant financial relationships.
Naval Vikram indicated no relevant financial relationships.
Sanjeev Sinha indicated no relevant financial relationships.
Ashutosh Biswas indicated no relevant financial relationships.
Naveet Wig indicated no relevant financial relationships.
Umang Arora, MBBS, MD1, Karan Sachdeva, MBBS1, Prerna Garg, MBBS, MD1, Upendra Baitha, MBBS, MD1, Saurabh Kedia, MBBS, MD, DM1, M Kalaivani, PhD1, Vineet Ahuja, MBBS, MD, DM2, Arvind Kumar, MBBS, MD1, Piyush Ranjan, MBBS, MD1, Naval K. Vikram, MBBS, MD1, Sanjeev Sinha, MBBS, MD1, Ashutosh Biswas, MBBS, MD1, Naveet Wig, MBBS, MD1. D0259 - Efficacy of Rifaximin in Patients With Abdominal Bloating or Distension: A Systematic Review and Meta-Analysis, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
