Back

Poster Session B - Monday Morning
Category: Interventional Endoscopy
B0481 - Removal of Orbera Intragastric Balloon Using Endoscopic Ultrasound
Monday, October 24, 2022
10:00 AM – 12:00 PM ET
Location: Crown Ballroom

Has Audio

Cynthia Cohen, MD
Westchester Medical Center
White Plains, New York
Presenting Author(s)
Cynthia Cohen, MD1, Dafna Somogyi, BS2, Jakob Saidman, MD, MS3, Shireen Pais, MD3
1Westchester Medical Center, White Plains, NY; 2New York Medical College, Valhalla, NY; 3Westchester Medical Center, Valhalla, NY
Introduction: The Orbera intragastric balloon [IGB] was FDA-approved in the United States in 2015 for management of obesity. The balloon is placed endoscopically and requires endoscopic removal within six months. Most patients who undergo IGB placement initially experience mild gastrointestinal symptoms such as nausea, vomiting, acid reflux, and abdominal pain due to gastric accommodation. Occasionally, early removal of the balloon is necessary due to intolerable symptoms, dehydration, gastric outlet obstruction, or infection. We present a novel case of IGB removal using endoscopic ultrasound [EUS]-guided aspiration.
Case Description/Methods: A twenty-four-year-old woman with obesity underwent Orbera IGB placement in the Dominican Republic without initial complication and lost forty-five pounds. Two months after IGB placement, she presented to the emergency room with two weeks of progressive epigastric pain, nausea, nonbilious but occasionally blood-streaked vomiting, inability to tolerate oral intake, and constipation. Vital signs were normal. Laboratory studies demonstrated hemoconcentration and low serum magnesium levels. She was also deficient in folate, iron, and vitamins A, D, and E. Abdominal radiograph demonstrated air and fluid in the stomach. She was admitted to the hospital for fluid resuscitation.
On hospital day two, she underwent esophagogastroduodenoscopy [EGD] and EUS with removal of the IGB. On initial EGD inspection, the Orbera balloon was identified in the stomach and the scope was able to pass beyond the balloon. The scope was withdrawn and then the EUS was performed. The balloon was identified and a nineteen-gauge echotip needle was introduced into balloon. Six hundred milliliters of methylene-blue colored fluid were aspirated. The EGD scope was reintroduced and the deflated balloon was removed with a grasper. On EGD inspection after balloon removal, continuous edema and erosion of the mucosa were seen in the fundus. Post-procedure, her symptoms resolved and she was discharged home.
Discussion: As the use of IGBs for obesity management becomes more common, it is important that endoscopists have the ability to safely remove them. Medical centers that do not specialize in bariatric endoscopy are unlikely to stock IGB removal kits. Other techniques such as puncturing the balloon with biopsy forceps or a needle knife are generally ineffective. EUS-guided aspiration of an IGB was successful in this case and can be easily replicated at any institution with a endoscopist proficient in EUS.

Disclosures:
Cynthia Cohen, MD1, Dafna Somogyi, BS2, Jakob Saidman, MD, MS3, Shireen Pais, MD3. B0481 - Removal of Orbera Intragastric Balloon Using Endoscopic Ultrasound, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1Westchester Medical Center, White Plains, NY; 2New York Medical College, Valhalla, NY; 3Westchester Medical Center, Valhalla, NY
Introduction: The Orbera intragastric balloon [IGB] was FDA-approved in the United States in 2015 for management of obesity. The balloon is placed endoscopically and requires endoscopic removal within six months. Most patients who undergo IGB placement initially experience mild gastrointestinal symptoms such as nausea, vomiting, acid reflux, and abdominal pain due to gastric accommodation. Occasionally, early removal of the balloon is necessary due to intolerable symptoms, dehydration, gastric outlet obstruction, or infection. We present a novel case of IGB removal using endoscopic ultrasound [EUS]-guided aspiration.
Case Description/Methods: A twenty-four-year-old woman with obesity underwent Orbera IGB placement in the Dominican Republic without initial complication and lost forty-five pounds. Two months after IGB placement, she presented to the emergency room with two weeks of progressive epigastric pain, nausea, nonbilious but occasionally blood-streaked vomiting, inability to tolerate oral intake, and constipation. Vital signs were normal. Laboratory studies demonstrated hemoconcentration and low serum magnesium levels. She was also deficient in folate, iron, and vitamins A, D, and E. Abdominal radiograph demonstrated air and fluid in the stomach. She was admitted to the hospital for fluid resuscitation.
On hospital day two, she underwent esophagogastroduodenoscopy [EGD] and EUS with removal of the IGB. On initial EGD inspection, the Orbera balloon was identified in the stomach and the scope was able to pass beyond the balloon. The scope was withdrawn and then the EUS was performed. The balloon was identified and a nineteen-gauge echotip needle was introduced into balloon. Six hundred milliliters of methylene-blue colored fluid were aspirated. The EGD scope was reintroduced and the deflated balloon was removed with a grasper. On EGD inspection after balloon removal, continuous edema and erosion of the mucosa were seen in the fundus. Post-procedure, her symptoms resolved and she was discharged home.
Discussion: As the use of IGBs for obesity management becomes more common, it is important that endoscopists have the ability to safely remove them. Medical centers that do not specialize in bariatric endoscopy are unlikely to stock IGB removal kits. Other techniques such as puncturing the balloon with biopsy forceps or a needle knife are generally ineffective. EUS-guided aspiration of an IGB was successful in this case and can be easily replicated at any institution with a endoscopist proficient in EUS.

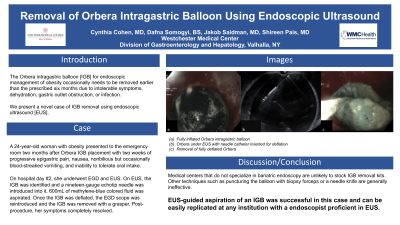
Figure: a. Fully inflated Orbera intragastric balloon
b. Orbera under EUS with needle catheter inserted for deflation
c. Removal of fully deflated Orbera
b. Orbera under EUS with needle catheter inserted for deflation
c. Removal of fully deflated Orbera
Disclosures:
Cynthia Cohen indicated no relevant financial relationships.
Dafna Somogyi indicated no relevant financial relationships.
Jakob Saidman indicated no relevant financial relationships.
Shireen Pais: abbvie – Speakers Bureau. boston scientific – Consultant. olympus – Consultant.
Cynthia Cohen, MD1, Dafna Somogyi, BS2, Jakob Saidman, MD, MS3, Shireen Pais, MD3. B0481 - Removal of Orbera Intragastric Balloon Using Endoscopic Ultrasound, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
