Back

Poster Session E - Tuesday Afternoon
Category: General Endoscopy
E0294 - All Roads Lead to SMAD4: Menetrier’s Disease in Association With a SMAD4 Mutation
Tuesday, October 25, 2022
3:00 PM – 5:00 PM ET
Location: Crown Ballroom

Has Audio

Yianni Protopapadakis, MD
Prisma Health, SC
Presenting Author(s)
Yianni Protopapadakis, MD1, Hubert Fenton, MD2, Christina Bauer, MD1, Veeral Oza, BS, MD1
1Prisma Health, Greenville, SC; 2Tenet Health, El Paso, TX
Introduction: Menetriere’s Disease (MD) is a rare protein losing enteropathy. A common histological feature of MD is massive foveolar hyperplasia (expansion of mucus cells). Medical therapies include Cetuximab and Octreotide but total gastrectomy remains the mainstay as a curative option. We report a case of MD, unrelated to infection in a patient with a germline SMAD4 mutation.
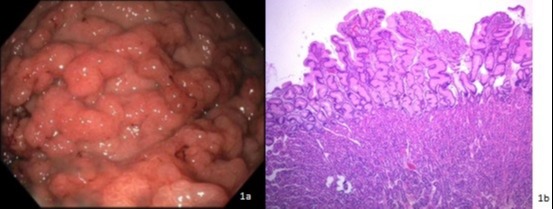
Case Description/Methods: A 39-year-old male with past medical history of hypertension, and an extensive orthopedic history was referred for iron deficiency anemia. Initial workup was notable for WBC 9,700cells/uL, Hemoglobin 6.2g/dL, Hct 23%, MCV 80.1 fL, Platelets 423 k/uL, Ferritin 13.2 ng/mL, Iron 40 ug/dL, total iron binding capacity 304 ug/dL, Transferrin 243 mg/dL, and transferrin saturation 12%. Symptoms included fatigue and exertional dyspnea but no gastrointestinal symptoms such as melena, hematochezia, abdominal pain, or diarrhea. Family history was notable for Juvenile Polyposis Syndrome (JPS) and a benign gastric mass removed by a partial gastrectomy in his father. He underwent endoscopic workup for further assessment. Upper endoscopy demonstrated severe gastritis and diffusely thickened gastric folds with thick mucus secretion in the body and cardia. [Figure 1a] The antrum appeared normal. Body and cardia biopsies showed diffuse foveolar hyperplasia with cystically dilated foveolar glands and edematous, mildly inflamed lamina propria consistent with MD. [Figure 1b] Biopsies were negative for H. Pylori and lacked viral cytopathic changes. Due to his family history of JPS, he was referred for genetic testing which revealed a SMAD4 gene mutation. The patient was admitted several weeks later for worsening anemia and renal insufficiency and diagnosed with atypical Hemolytic Uremic Syndrome and unfortunately passed away from complications
Discussion: SMAD4 is an intracellular signaling mediator of the Transforming growth factor beta (TGF-β) pathway. Loss of TGF- β stimulation leads to unopposed TGF-a processes, causing features consistent with MD (decreased stomach acidity, hyperplasia of mucus cells, and gastric antralization). Only a handful of documented associations have been published between MD and SMAD4. In one study, with overlap of MD and JPS, SMAD4 was hypothesized to be the causative mutation for both. Despite lacking confirmatory genetic testing in our patient’s father, it is safe to assume the autosomal dominant SMAD4 mutation is the culprit in both individuals despite the differential expression as disease.
Disclosures:
Yianni Protopapadakis, MD1, Hubert Fenton, MD2, Christina Bauer, MD1, Veeral Oza, BS, MD1. E0294 - All Roads Lead to SMAD4: Menetrier’s Disease in Association With a SMAD4 Mutation, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1Prisma Health, Greenville, SC; 2Tenet Health, El Paso, TX
Introduction: Menetriere’s Disease (MD) is a rare protein losing enteropathy. A common histological feature of MD is massive foveolar hyperplasia (expansion of mucus cells). Medical therapies include Cetuximab and Octreotide but total gastrectomy remains the mainstay as a curative option. We report a case of MD, unrelated to infection in a patient with a germline SMAD4 mutation.
Case Description/Methods: A 39-year-old male with past medical history of hypertension, and an extensive orthopedic history was referred for iron deficiency anemia. Initial workup was notable for WBC 9,700cells/uL, Hemoglobin 6.2g/dL, Hct 23%, MCV 80.1 fL, Platelets 423 k/uL, Ferritin 13.2 ng/mL, Iron 40 ug/dL, total iron binding capacity 304 ug/dL, Transferrin 243 mg/dL, and transferrin saturation 12%. Symptoms included fatigue and exertional dyspnea but no gastrointestinal symptoms such as melena, hematochezia, abdominal pain, or diarrhea. Family history was notable for Juvenile Polyposis Syndrome (JPS) and a benign gastric mass removed by a partial gastrectomy in his father. He underwent endoscopic workup for further assessment. Upper endoscopy demonstrated severe gastritis and diffusely thickened gastric folds with thick mucus secretion in the body and cardia. [Figure 1a] The antrum appeared normal. Body and cardia biopsies showed diffuse foveolar hyperplasia with cystically dilated foveolar glands and edematous, mildly inflamed lamina propria consistent with MD. [Figure 1b] Biopsies were negative for H. Pylori and lacked viral cytopathic changes. Due to his family history of JPS, he was referred for genetic testing which revealed a SMAD4 gene mutation. The patient was admitted several weeks later for worsening anemia and renal insufficiency and diagnosed with atypical Hemolytic Uremic Syndrome and unfortunately passed away from complications
Discussion: SMAD4 is an intracellular signaling mediator of the Transforming growth factor beta (TGF-β) pathway. Loss of TGF- β stimulation leads to unopposed TGF-a processes, causing features consistent with MD (decreased stomach acidity, hyperplasia of mucus cells, and gastric antralization). Only a handful of documented associations have been published between MD and SMAD4. In one study, with overlap of MD and JPS, SMAD4 was hypothesized to be the causative mutation for both. Despite lacking confirmatory genetic testing in our patient’s father, it is safe to assume the autosomal dominant SMAD4 mutation is the culprit in both individuals despite the differential expression as disease.

Figure: Endoscopic and Pathologic appearance of Menetriers Disease associated with SMAD4
Disclosures:
Yianni Protopapadakis indicated no relevant financial relationships.
Hubert Fenton indicated no relevant financial relationships.
Christina Bauer indicated no relevant financial relationships.
Veeral Oza: Boston Scientific – Consultant. S4 Medical – Advisor or Review Panel Member, Advisory Committee/Board Member.
Yianni Protopapadakis, MD1, Hubert Fenton, MD2, Christina Bauer, MD1, Veeral Oza, BS, MD1. E0294 - All Roads Lead to SMAD4: Menetrier’s Disease in Association With a SMAD4 Mutation, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.

