Back
Poster Session E - Tuesday Afternoon
Category: IBD
E0354 - Association Between Absolute Lymphocyte Count and Ozanimod Efficacy/Safety in Patients With Moderate/Severe Ulcerative Colitis: Results From the Phase 3 True North Study
Tuesday, October 25, 2022
3:00 PM – 5:00 PM ET
Location: Crown Ballroom

- SH
Sarah Harris, PhD
Bristol Myers Squibb
Princeton, New Jersey
Presenting Author(s)
Sarah Harris, PhD, Rachel Maddux, PhD, Chun Wu, PhD, Sarah Hu, PhD, AnnKatrin Petersen, MD, MS
Bristol Myers Squibb, Princeton, NJ
Introduction: Ozanimod (OZA) is approved in the USA and EU for treatment (tx) of moderate/severe UC. OZA binding internalizes S1P1 receptors, reducing egress of lymphocyte subsets into circulation. OZA was approved in this patient (pt) population based on results of the phase 3 True North (TN) study (NCT02435992).
Methods: We evaluated association between absolute lymphocyte count (ALC) and OZA efficacy/safety during TN’s induction period (IP) and maintenance period (MP). Pts were randomized to double-blind (DB) OZA 0.92 mg or placebo (PBO) or open-label (OL) OZA during 10-week (W) IP. Pts with clinical response to OZA at W10 were rerandomized to DB OZA or PBO for MP through W52. Rectal bleeding (RB), stool frequency (SF), composite Mayo, Physician’s Global Assessment (PGA), and endoscopy scores were assessed at baseline and as clinical outcomes at W10 and W52. Efficacy endpoints (EPs; clinical remission, clinical response, endoscopic improvement, mucosal healing, and histologic remission) were assessed at W10 and W52. Tx-emergent adverse events (TEAEs) and ALC were evaluated at baseline and W5, W10, W18, W28, W40, and W52.
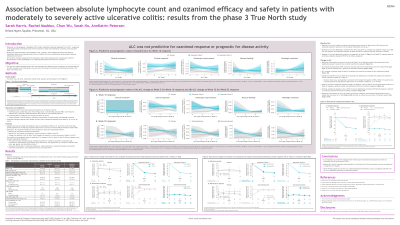
Results: During IP, 645 pts received DB OZA (n=429) or PBO (n=216) and 367 received OL OZA; for MP, 230 and 227 OZA-treated pts were rerandomized to OZA and PBO, respectively. Baseline ALC was not significantly correlated with clinical outcome at W10 or W52 as measured by changes from baseline RB, SF, Mayo, PGA, and endoscopy scores in pts on OZA or PBO. Baseline ALC was not predictive or prognostic for W10 and W52 responses based on the efficacy EPs. Reductions from baseline in mean ALC occurred by W5 with OZA and were significantly greater with OZA (53-54%) vs PBO (2.3%; P< .001). ALC plateaued by W10 and was maintained through W52 in pts on continuous OZA. ALC returned to PBO levels by W52 in pts who switched to PBO during MP. Change in ALC at W10 was generally not significantly correlated with changes in clinical outcomes at W10 or W52. Change in ALC at W5 was not predictive of W10 response; change in ALC at W10 was not predictive of W52 response. Reductions from baseline in ALC at all weeks were similar in OZA-treated pts with/without ≥1 TEAE and with/without infection TEAEs.
Discussion: ALC reductions occurring with OZA were reversed upon tx discontinuation and were not associated with TEAEs. Baseline ALC and ALC reductions were not predictive or prognostic for response. These findings support ALC as a pharmacodynamic biomarker but not as a prognostic or predictive biomarker.
Disclosures:
Sarah Harris, PhD, Rachel Maddux, PhD, Chun Wu, PhD, Sarah Hu, PhD, AnnKatrin Petersen, MD, MS. E0354 - Association Between Absolute Lymphocyte Count and Ozanimod Efficacy/Safety in Patients With Moderate/Severe Ulcerative Colitis: Results From the Phase 3 True North Study, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
Bristol Myers Squibb, Princeton, NJ
Introduction: Ozanimod (OZA) is approved in the USA and EU for treatment (tx) of moderate/severe UC. OZA binding internalizes S1P1 receptors, reducing egress of lymphocyte subsets into circulation. OZA was approved in this patient (pt) population based on results of the phase 3 True North (TN) study (NCT02435992).
Methods: We evaluated association between absolute lymphocyte count (ALC) and OZA efficacy/safety during TN’s induction period (IP) and maintenance period (MP). Pts were randomized to double-blind (DB) OZA 0.92 mg or placebo (PBO) or open-label (OL) OZA during 10-week (W) IP. Pts with clinical response to OZA at W10 were rerandomized to DB OZA or PBO for MP through W52. Rectal bleeding (RB), stool frequency (SF), composite Mayo, Physician’s Global Assessment (PGA), and endoscopy scores were assessed at baseline and as clinical outcomes at W10 and W52. Efficacy endpoints (EPs; clinical remission, clinical response, endoscopic improvement, mucosal healing, and histologic remission) were assessed at W10 and W52. Tx-emergent adverse events (TEAEs) and ALC were evaluated at baseline and W5, W10, W18, W28, W40, and W52.
Results: During IP, 645 pts received DB OZA (n=429) or PBO (n=216) and 367 received OL OZA; for MP, 230 and 227 OZA-treated pts were rerandomized to OZA and PBO, respectively. Baseline ALC was not significantly correlated with clinical outcome at W10 or W52 as measured by changes from baseline RB, SF, Mayo, PGA, and endoscopy scores in pts on OZA or PBO. Baseline ALC was not predictive or prognostic for W10 and W52 responses based on the efficacy EPs. Reductions from baseline in mean ALC occurred by W5 with OZA and were significantly greater with OZA (53-54%) vs PBO (2.3%; P< .001). ALC plateaued by W10 and was maintained through W52 in pts on continuous OZA. ALC returned to PBO levels by W52 in pts who switched to PBO during MP. Change in ALC at W10 was generally not significantly correlated with changes in clinical outcomes at W10 or W52. Change in ALC at W5 was not predictive of W10 response; change in ALC at W10 was not predictive of W52 response. Reductions from baseline in ALC at all weeks were similar in OZA-treated pts with/without ≥1 TEAE and with/without infection TEAEs.
Discussion: ALC reductions occurring with OZA were reversed upon tx discontinuation and were not associated with TEAEs. Baseline ALC and ALC reductions were not predictive or prognostic for response. These findings support ALC as a pharmacodynamic biomarker but not as a prognostic or predictive biomarker.
Disclosures:
Sarah Harris: Bristol Myers Squibb – Employee, Stock-publicly held company(excluding mutual/index funds).
Rachel Maddux: Bristol Myers Squibb – Employee.
Chun Wu: Bristol Myers Squibb – Employee, Stock-publicly held company(excluding mutual/index funds).
Sarah Hu: Bristol Myers Squibb – Employee, Stock-publicly held company(excluding mutual/index funds).
AnnKatrin Petersen: Bristol Myers Squibb – Employee, Stock-publicly held company(excluding mutual/index funds).
Sarah Harris, PhD, Rachel Maddux, PhD, Chun Wu, PhD, Sarah Hu, PhD, AnnKatrin Petersen, MD, MS. E0354 - Association Between Absolute Lymphocyte Count and Ozanimod Efficacy/Safety in Patients With Moderate/Severe Ulcerative Colitis: Results From the Phase 3 True North Study, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
