Back

Poster Session D - Tuesday Morning
Category: Liver
D0531 - Drug-Induced Liver Injury by Novel Dietary Supplement
Tuesday, October 25, 2022
10:00 AM – 12:00 PM ET
Location: Crown Ballroom

Has Audio

Matthew Kobeszko, MD, MBA, MS
Advocate Aurora Health
Milwaukee, WI
Presenting Author(s)
Matthew Kobeszko, MD, MBA, MS, Rehana Begum, MD
Advocate Aurora Health, Milwaukee, WI
Introduction: Drug-induced liver injury (DILI) secondary to herbal and dietary supplements present with unique diagnostic challenges with potentially devastating clinical outcomes if left untreated. We present a case of biopsy confirmed drug induced liver injury secondary to ningxia wolfberry puree. To our knowledge, this is the first case report in literature.
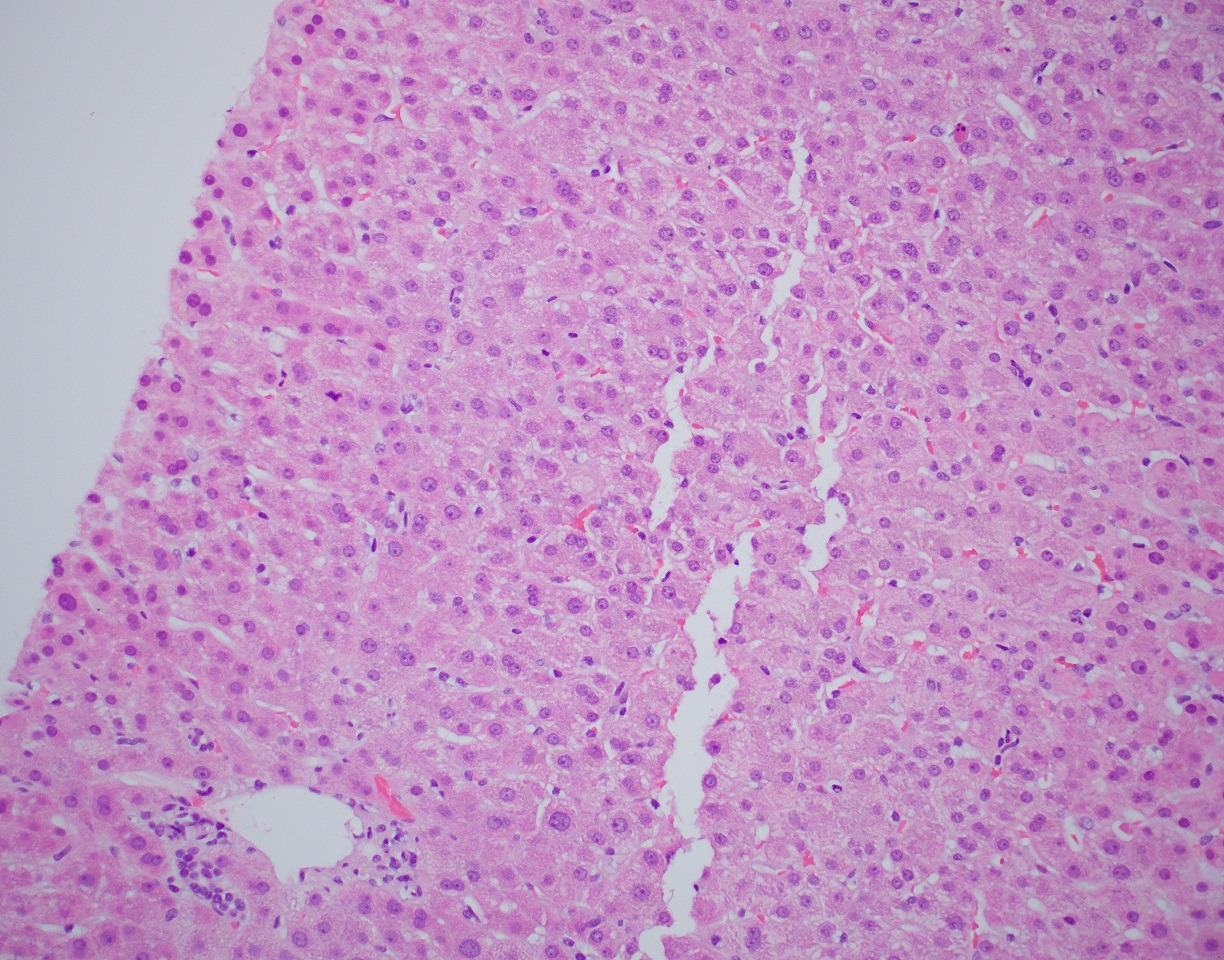
Case Description/Methods: A 60-year-old female with no prior medical history presented to an outpatient clinic for evaluation of jaundice, pruritis, and a cutaneous rash. Patient was not previously taking any prescription medications and the only recent change was the addition of a new supplement containing ningxia wolfberry puree 6 weeks prior. Liver enzymes were elevated with total bilirubin 7.5, direct bilirubin 5.8, ALT 161, AST 381, and alkaline phosphatase 304. Patient underwent a chronic liver disease work up that was negative including viral, autoimmune, and metabolic etiologies. MRCP demonstrated no common bile duct dilation or biliary obstruction. Liver biopsy demonstrated intracanalicular cholestasis and lobular inflammation (image 1). Liver enzymes continued to up-trend at which point she was started on prednisone with a subsequent taper. Liver enzymes reached normalization over the course of 5 weeks. A 3-month follow up after treatment and supplement discontinuation demonstrated continued normal liver enzymes.
Discussion: The growth of over-the-counter supplement use has begun to present diagnostic challenges of identifying accurate diagnosis and management. While prescription medications undergo scrutinous review, most dietary and herbal supplements are classified as a food product. Therefore, the Food and Drug Administration does not require provisional review for safety. The worldwide incidence of DILI reported as 19 per 100,000 with 16 percent attributed to dietary supplements. In addition, DILI secondary to supplements accounts for 11% of acute liver failure cases overall, with a mortality rate of 8% and 2% requiring a transplantation. Even with prompt diagnosis and management, studies have demonstrated 14% of individuals will have continued liver test abnormalities beyond 6 months. While discontinuation of the offending agent is typically sufficient, more severe cases require further treatment with prednisone or n-acetyl cysteine. While many supplements are advertised as having antioxidant and immunomodulatory activity, many of these may contain ingredients that have strong biological effects with unclear acute and chronic risks.
Disclosures:
Matthew Kobeszko, MD, MBA, MS, Rehana Begum, MD. D0531 - Drug-Induced Liver Injury by Novel Dietary Supplement, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
Advocate Aurora Health, Milwaukee, WI
Introduction: Drug-induced liver injury (DILI) secondary to herbal and dietary supplements present with unique diagnostic challenges with potentially devastating clinical outcomes if left untreated. We present a case of biopsy confirmed drug induced liver injury secondary to ningxia wolfberry puree. To our knowledge, this is the first case report in literature.
Case Description/Methods: A 60-year-old female with no prior medical history presented to an outpatient clinic for evaluation of jaundice, pruritis, and a cutaneous rash. Patient was not previously taking any prescription medications and the only recent change was the addition of a new supplement containing ningxia wolfberry puree 6 weeks prior. Liver enzymes were elevated with total bilirubin 7.5, direct bilirubin 5.8, ALT 161, AST 381, and alkaline phosphatase 304. Patient underwent a chronic liver disease work up that was negative including viral, autoimmune, and metabolic etiologies. MRCP demonstrated no common bile duct dilation or biliary obstruction. Liver biopsy demonstrated intracanalicular cholestasis and lobular inflammation (image 1). Liver enzymes continued to up-trend at which point she was started on prednisone with a subsequent taper. Liver enzymes reached normalization over the course of 5 weeks. A 3-month follow up after treatment and supplement discontinuation demonstrated continued normal liver enzymes.
Discussion: The growth of over-the-counter supplement use has begun to present diagnostic challenges of identifying accurate diagnosis and management. While prescription medications undergo scrutinous review, most dietary and herbal supplements are classified as a food product. Therefore, the Food and Drug Administration does not require provisional review for safety. The worldwide incidence of DILI reported as 19 per 100,000 with 16 percent attributed to dietary supplements. In addition, DILI secondary to supplements accounts for 11% of acute liver failure cases overall, with a mortality rate of 8% and 2% requiring a transplantation. Even with prompt diagnosis and management, studies have demonstrated 14% of individuals will have continued liver test abnormalities beyond 6 months. While discontinuation of the offending agent is typically sufficient, more severe cases require further treatment with prednisone or n-acetyl cysteine. While many supplements are advertised as having antioxidant and immunomodulatory activity, many of these may contain ingredients that have strong biological effects with unclear acute and chronic risks.

Figure: Liver Biopsy
Disclosures:
Matthew Kobeszko indicated no relevant financial relationships.
Rehana Begum indicated no relevant financial relationships.
Matthew Kobeszko, MD, MBA, MS, Rehana Begum, MD. D0531 - Drug-Induced Liver Injury by Novel Dietary Supplement, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.

