Back

Poster Session A - Sunday Afternoon
Category: General Endoscopy
A0275 - Sevelamer: An Underreported Cause of Enteritis and Colitis
Sunday, October 23, 2022
5:00 PM – 7:00 PM ET
Location: Crown Ballroom

Has Audio
- WG
Willie C. Gilbert, DO
Northside Hospital Gwinnett
Lawrenceville, Georgia
Presenting Author(s)
Willie C. Gilbert, DO1, Thuy-Van P. Hang, MD2, Jigna Jani, MD3, Anand Shah, MD4
1Northside Hospital Gwinnett, Lawrenceville, GA; 2Emory University School of Medicine, Atlanta, GA; 3Atlanta VA, Decatur, GA; 4Emory University School of Medicine, Decatur, GA
Introduction: Hyperphosphatemia is a common metabolic derangement in patients with end-stage renal disease (ESRD). In addition to dietary constraints, phosphate binders are needed to treat hyperphosphatemia. Sevelamer is the most used resin-based phosphate binder and can crystallize to form concretions. Crystals formed by other resin-based binders such as polystyrene sulfonate (kayexalate) are a well-known cause of gastrointestinal (GI) mucosal injury. However, sevelamer is an underreported culprit. Here, we describe a case of ileitis and colitis caused by sevelamer crystals.
Case Description/Methods: A 65-year-old male veteran with ESRD, secondary hyperparathyroidism treated with sevelamer, and cocaine abuse was admitted for abdominal pain and bloody diarrhea for three days. CT scan of the abdomen and pelvis revealed inflammation of the ileum and rectosigmoid. He was started on ciprofloxacin and metronidazole. Stool studies were negative. A colonoscopy revealed an ulcerated and erythematous terminal ileum (TI) between 15cm and 7cm proximal to the ileocecal valve. There was mild ulceration and erythema in the ascending colon. The worst ulceration was between 40cm to 30cm from the anal verge with additional ulceration seen in the rectosigmoid. The colonic mucosa between the ulcerations were normal appearing. Biopsies taken from the TI, ascending colon, and descending colon revealed chronic ileitis and colitis with ulceration, granulation tissue associated crystals, crypt abscess, and fibrinopurulent debris and negative for infection or malignancy. A review the medication reconciliation confirmed no exposure to kayexalate and, thus, concluded that sevelamer was the cause. The patient was advised to stop taking sevelamer. A follow up colonoscopy six months later revealed complete mucosal healing.
Discussion: GI mucosal injury is a rare and underrecognized adverse effect of sevelamer. A limited number of reported cases describe a range of symptoms such as nausea, vomiting, constipation, dysentery, and acute abdomen requiring surgery. The enteritis and colitis caused by sevelamer can be overlooked and presumed to be infectious or ischemic etiology. The pathology can also be missed because of misidentification or failure to identify crystals on histology. Increased awareness of this underreported complication of sevelamer is important for these reasons and for directing appropriate therapy with cessation of this medication in the setting of enteritis or colitis.
Disclosures:
Willie C. Gilbert, DO1, Thuy-Van P. Hang, MD2, Jigna Jani, MD3, Anand Shah, MD4. A0275 - Sevelamer: An Underreported Cause of Enteritis and Colitis, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1Northside Hospital Gwinnett, Lawrenceville, GA; 2Emory University School of Medicine, Atlanta, GA; 3Atlanta VA, Decatur, GA; 4Emory University School of Medicine, Decatur, GA
Introduction: Hyperphosphatemia is a common metabolic derangement in patients with end-stage renal disease (ESRD). In addition to dietary constraints, phosphate binders are needed to treat hyperphosphatemia. Sevelamer is the most used resin-based phosphate binder and can crystallize to form concretions. Crystals formed by other resin-based binders such as polystyrene sulfonate (kayexalate) are a well-known cause of gastrointestinal (GI) mucosal injury. However, sevelamer is an underreported culprit. Here, we describe a case of ileitis and colitis caused by sevelamer crystals.
Case Description/Methods: A 65-year-old male veteran with ESRD, secondary hyperparathyroidism treated with sevelamer, and cocaine abuse was admitted for abdominal pain and bloody diarrhea for three days. CT scan of the abdomen and pelvis revealed inflammation of the ileum and rectosigmoid. He was started on ciprofloxacin and metronidazole. Stool studies were negative. A colonoscopy revealed an ulcerated and erythematous terminal ileum (TI) between 15cm and 7cm proximal to the ileocecal valve. There was mild ulceration and erythema in the ascending colon. The worst ulceration was between 40cm to 30cm from the anal verge with additional ulceration seen in the rectosigmoid. The colonic mucosa between the ulcerations were normal appearing. Biopsies taken from the TI, ascending colon, and descending colon revealed chronic ileitis and colitis with ulceration, granulation tissue associated crystals, crypt abscess, and fibrinopurulent debris and negative for infection or malignancy. A review the medication reconciliation confirmed no exposure to kayexalate and, thus, concluded that sevelamer was the cause. The patient was advised to stop taking sevelamer. A follow up colonoscopy six months later revealed complete mucosal healing.
Discussion: GI mucosal injury is a rare and underrecognized adverse effect of sevelamer. A limited number of reported cases describe a range of symptoms such as nausea, vomiting, constipation, dysentery, and acute abdomen requiring surgery. The enteritis and colitis caused by sevelamer can be overlooked and presumed to be infectious or ischemic etiology. The pathology can also be missed because of misidentification or failure to identify crystals on histology. Increased awareness of this underreported complication of sevelamer is important for these reasons and for directing appropriate therapy with cessation of this medication in the setting of enteritis or colitis.

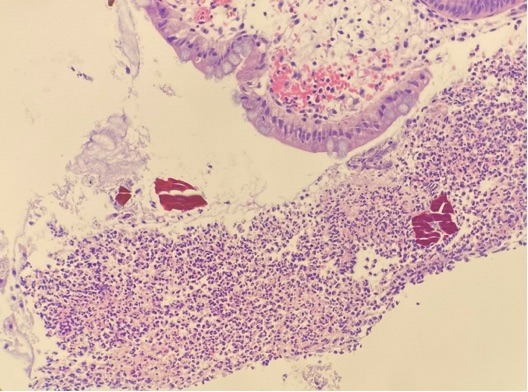
Figure: H&E stain showing fibrinopurulent exudate (ischemic /acute erosive pattern colitis ) and the presence of Sevelamer crystals.
Disclosures:
Willie Gilbert indicated no relevant financial relationships.
Thuy-Van Hang indicated no relevant financial relationships.
Jigna Jani indicated no relevant financial relationships.
Anand Shah indicated no relevant financial relationships.
Willie C. Gilbert, DO1, Thuy-Van P. Hang, MD2, Jigna Jani, MD3, Anand Shah, MD4. A0275 - Sevelamer: An Underreported Cause of Enteritis and Colitis, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
