Back

Poster Session E - Tuesday Afternoon
Category: Liver
E0500 - Increasing Hepatitis C Virus Screening Across Inner City Community Clinics: A Quality Improvement Project
Tuesday, October 25, 2022
3:00 PM – 5:00 PM ET
Location: Crown Ballroom

Has Audio
- MS
Mantej Sehmbhi, MBBS, MSc, MRCP
Mount Sinai Morningside and West Hospitals
New York, NY
Presenting Author(s)
Mantej Sehmbhi, MBBS, MSc, MRCP1, Emily Seltzer, DO, MS2, Nour Al Khalili, MD3, Shabari M. Shenoy, MBBS2, Patricia Miguez Arosemena, MD1, Geeta Varghese, MD1
1Mount Sinai Morningside and West Hospitals, New York, NY; 2Icahn School of Medicine at Mount Sinai Morningside-West, New York, NY; 3Mount Sinai Morningside/West, New York, NY
Introduction: Hepatitis C virus (HCV) infection is a chronic infection in the majority of patients, with important sequelae including the development of cirrhosis and hepatocellular carcinoma (HCC). HCV treatments are effective at reducing cirrhosis and HCC rates, but an estimated 51% of those infected with HCV are not aware of the diagnosis. The United States Preventive Services Task force (USPSTF) changed its HCV screening guidelines in 2020 to expand eligibility for screening to all those aged 18 to 79 years. We conducted a quality improvement (QI) project at three federally-funded primary care clinic sites in New York City, to raise awareness of the change in USPSTF guidelines and to improve screening rates.
Methods: We utilized the Plan-Do-Study-Act (PDSA) QI methodology for this project. We gathered pre-intervention data on which patients had a HCV screening antibody test logged in the electronic medical record, stratified by clinic site. Surveys were sent out to resident physicians to assess awareness of HCV presentation, treatment and screening guidelines. We deployed tailored interventions aimed at increasing awareness among both providers and patients at each site, including regular educational sessions for staff, informational posters in waiting areas, and reminders in consultation rooms. Interventions were implemented throughout the six-month period from August 2021 to January 2022. We gathered post-intervention data at 3 and 6 months to examine changes in screening rates.
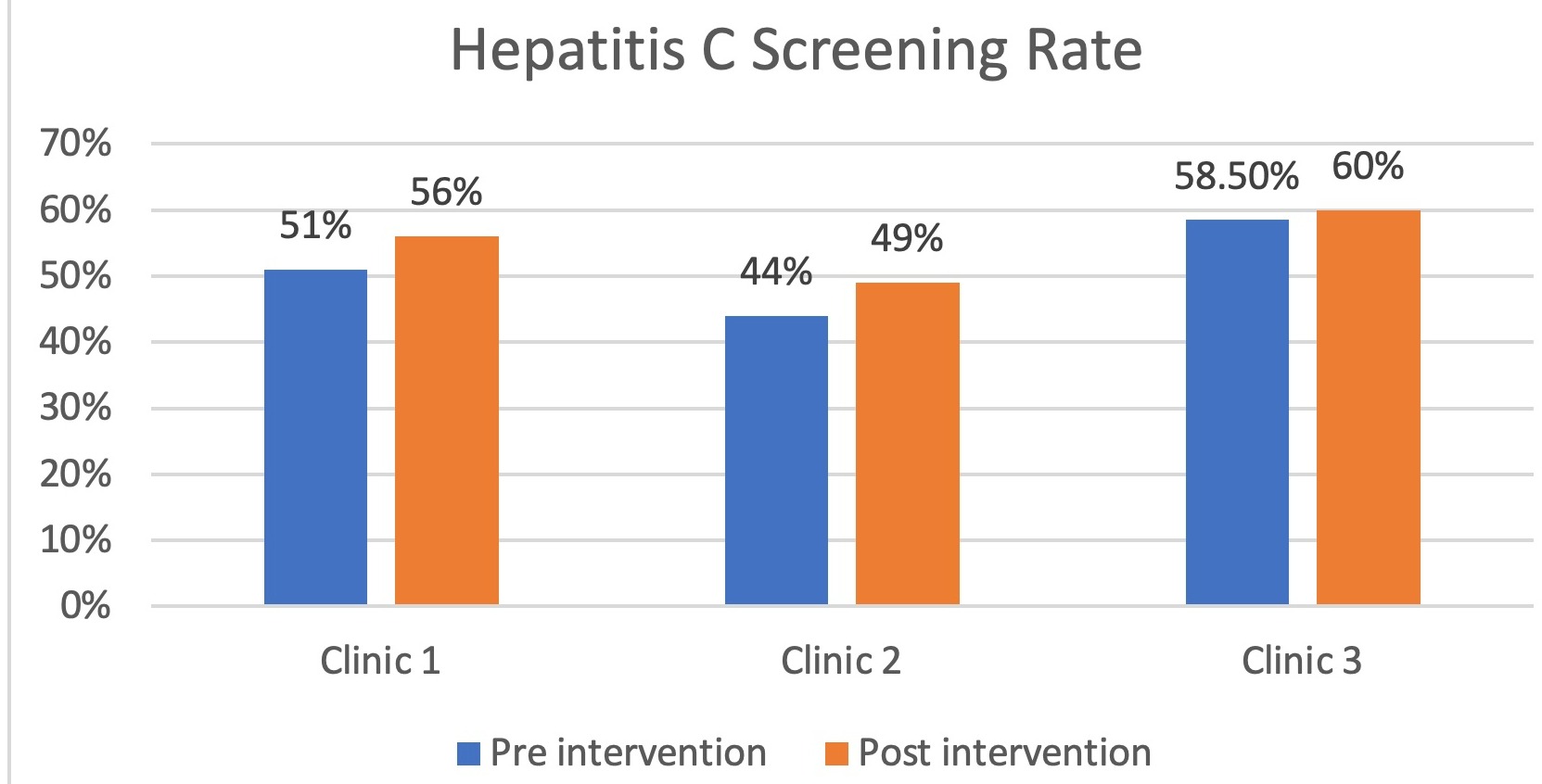
Results: Pre-intervention, we found that HCV screening rates were moderate at all sites: 51% of adults at Clinic 1, 44% at Clinic 2, and 58.5% at Clinic 3 had at least one HCV antibody screen on record as of July 2021. However, 55% of residents surveyed were not aware of the recent changes in USPSTF guidelines. After implementation of the interventions described above, screening rates had increased to 56% at Clinic 1, 49% at Clinic 2 and 60% at Clinic 3 by February 2022.
Discussion: HCV screening rates at our sites, serving primarily underinsured patients in New York City, were higher than the 8-18% national average estimated by the USPSTF. Intensive education about screening guidelines to providers and patients was moderately effective at increasing screening rates at all three sites, though with variation in effect size. PDSA cycles continue, with interventions including altering workflow and daily reminders for providers. Further work is underway to understand reasons for lack of screening uptake.
Disclosures:
Mantej Sehmbhi, MBBS, MSc, MRCP1, Emily Seltzer, DO, MS2, Nour Al Khalili, MD3, Shabari M. Shenoy, MBBS2, Patricia Miguez Arosemena, MD1, Geeta Varghese, MD1. E0500 - Increasing Hepatitis C Virus Screening Across Inner City Community Clinics: A Quality Improvement Project, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1Mount Sinai Morningside and West Hospitals, New York, NY; 2Icahn School of Medicine at Mount Sinai Morningside-West, New York, NY; 3Mount Sinai Morningside/West, New York, NY
Introduction: Hepatitis C virus (HCV) infection is a chronic infection in the majority of patients, with important sequelae including the development of cirrhosis and hepatocellular carcinoma (HCC). HCV treatments are effective at reducing cirrhosis and HCC rates, but an estimated 51% of those infected with HCV are not aware of the diagnosis. The United States Preventive Services Task force (USPSTF) changed its HCV screening guidelines in 2020 to expand eligibility for screening to all those aged 18 to 79 years. We conducted a quality improvement (QI) project at three federally-funded primary care clinic sites in New York City, to raise awareness of the change in USPSTF guidelines and to improve screening rates.
Methods: We utilized the Plan-Do-Study-Act (PDSA) QI methodology for this project. We gathered pre-intervention data on which patients had a HCV screening antibody test logged in the electronic medical record, stratified by clinic site. Surveys were sent out to resident physicians to assess awareness of HCV presentation, treatment and screening guidelines. We deployed tailored interventions aimed at increasing awareness among both providers and patients at each site, including regular educational sessions for staff, informational posters in waiting areas, and reminders in consultation rooms. Interventions were implemented throughout the six-month period from August 2021 to January 2022. We gathered post-intervention data at 3 and 6 months to examine changes in screening rates.
Results: Pre-intervention, we found that HCV screening rates were moderate at all sites: 51% of adults at Clinic 1, 44% at Clinic 2, and 58.5% at Clinic 3 had at least one HCV antibody screen on record as of July 2021. However, 55% of residents surveyed were not aware of the recent changes in USPSTF guidelines. After implementation of the interventions described above, screening rates had increased to 56% at Clinic 1, 49% at Clinic 2 and 60% at Clinic 3 by February 2022.
Discussion: HCV screening rates at our sites, serving primarily underinsured patients in New York City, were higher than the 8-18% national average estimated by the USPSTF. Intensive education about screening guidelines to providers and patients was moderately effective at increasing screening rates at all three sites, though with variation in effect size. PDSA cycles continue, with interventions including altering workflow and daily reminders for providers. Further work is underway to understand reasons for lack of screening uptake.

Figure: Bar chart showing pre- and post-intervention HCV screening rates.
Disclosures:
Mantej Sehmbhi indicated no relevant financial relationships.
Emily Seltzer indicated no relevant financial relationships.
Nour Al Khalili indicated no relevant financial relationships.
Shabari Shenoy indicated no relevant financial relationships.
Patricia Miguez Arosemena indicated no relevant financial relationships.
Geeta Varghese indicated no relevant financial relationships.
Mantej Sehmbhi, MBBS, MSc, MRCP1, Emily Seltzer, DO, MS2, Nour Al Khalili, MD3, Shabari M. Shenoy, MBBS2, Patricia Miguez Arosemena, MD1, Geeta Varghese, MD1. E0500 - Increasing Hepatitis C Virus Screening Across Inner City Community Clinics: A Quality Improvement Project, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
