Poster Session E - Tuesday Afternoon
Category: Liver
E0502 - Progress in Drug Therapy for Hepatorenal Syndrome: A Systematic Review of Clinical Studies in the Last 3 Years

Mohammad Hamza, MD
Albany Medical Center Hospital
Aldan, PA
Presenting Author(s)
1Albany Medical Center Hospital, Albany, NY; 2University of South Dakota Sanford School of Medicine, Vermillion, SD; 3Dow University of Health Sciences, Karachi, Sindh, Pakistan; 4De la Salle Health Sciences Institute, Dasmariñas, Cavite, Philippines; 5Shifa College of Medicine, Shifa Tameer-e-Millat University, Rawalpindi, Punjab, Pakistan; 6Saint Michael's Medical Center, Newark, NJ; 7BronxCare Health System, Bronx, NY; 8Islamic International Medical College, Islamabad, Islamabad, Pakistan; 9Odessa National Medical University, Odessa, Odes'ka Oblast', Ukraine; 10Nottingham University Hospitals NHS, Nottingham, England, United Kingdom; 11Saint Clare’s Denville Hospital & St. Mary's General Hospital, Danville, NJ
Introduction: Rapid deterioration of kidneys in patients with severe liver injury i-e., cirrhosis is termed a hepatorenal syndrome (HRS). Hemodynamic instability due to increased splanchnic blood flow, systemic vasodilation, and renal vasoconstriction might cause HRS, and the goal of the drug therapies is to improve systematic circulation. This article aims to review the efficacy and safety of drug therapies tested in the last 3 years in HRS patients.
Methods: We searched PubMed, Embase, Cochrane, and WOS from 1/1/2019 till 05/15/2022. We screened 520 articles and included 3 clinical trials (N=462) and 5 observational studies (N=1,034) with >5 patients providing data about the safety and efficacy of drugs. All case reports, case series, review articles, meta-analyses, and clinical studies with irrelevant populations were excluded.
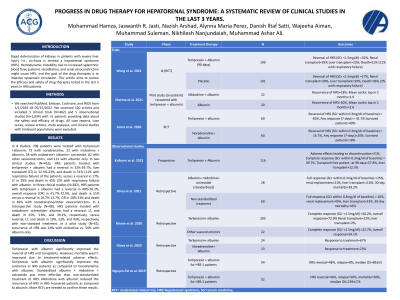
Results: In 8 studies, 708 patients were treated with terlipressin +albumin, 73 with noradrenaline, 22 with midodrine + albumin, 28 with midodrine+ albumin+ octreotide, 82 with other vasoconstrictors, and 121 with albumin only. In two clinical studies (N=416), HRS patients treated with terlipressin + albumin had a reversal in 32%-39.7%, liver transplant (LT) in 12.5%-23%, and death in 51% (11% with respiratory failure) of the patients, versus a reversal in 17%, LT in 29% and death in 45% (2% with respiratory failure) with albumin. In three clinical studies (N=382), HRS patients with terlipressin + albumin had a reversal in 40%-50.2%, overall response (OR) in 41.7%-72.9%, and death in 51% versus a reversal in 16.7%-22.7%, OR in 20%-23% and death in 82% with noradrenaline/other vasoconstrictors. In a retrospective study (N=88), HRS patients treated with midodrine+ octreotide+ albumin had a reversal, LT, and death in 25%, 3.6%, and 39.2%, respectively, versus reversal, LT, and death in 10%, 23%, and 43%, respectively, with non-standard treatment. In a pilot study (N=42), recurrence of HRS was 18% with midodrine vs. 50% with albumin only.
Discussion: Terlipressin with albumin significantly improved the reversal of HRS and transplants. However, mortality wasn’t improved due to treatment-related adverse effects. Terlipressin with albumin significantly improved the outcomes in HRS patients as compared to noradrenaline with albumin. Standardized albumin + midodrine + octreotide was more effective than non-standardized treatment of HRS. Midodrine with albumin reduced the recurrence of HRS in HRS recovered patients as compared to albumin. More RCTs are needed to confirm these results.
Study | Phase | Treatment therapy | N | Outcomes |
Trials | ||||
Wong et al. 2021 | III (RCT) | Terlipressin + Albumin (90 days) | 199 | Reversal of HRS (SCr < 1.5mg/dl) =32%, Renal transplant=29% Liver transplant=23%, Death=51% (11% with respiratory failure) |
Placebo | 101 | Reversal of HRS (SCr < 1.5mg/dl) =17%, Renal transplant=39%, Liver transplant=29%, Death=45% (2% with respiratory failure) | ||
Sharma et al. 2021 | Pilot study (on patients recovered with terlipressin + albumin) | Midodrine + albumin | 22 | Recurrence of HRS=18%, Mean ascitic tap in 2 months=1.9 |
Albumin | 20 | Recurrence of HRS=50%, Mean ascitic tap in 2 months=2.6 | ||
Arora et al. 2020 | RCT | Terlipressin + albumin | 60 | Reversal of HRS (SCr within 0.3mg/dl of baseline) = 40%, Any response (7-days) = 41.7% Survived patients=49% |
Noradrenaline+ albumin | 60 | Reversal of HRS (SCr within 0.3mg/dl of baseline) = 16.7%, Any response (7-days)=20%, Survived patients=18% | ||
Observational studies | ||||
Kulkarni et al. 2022 | Prospective | Terlipressin + Albumin | 116 | Adverse effects leading to discontinuation=21%, Complete response (SCr within 0.3mg/dl of baseline) = 39.7%, Transplant free patient -at 90 days=57.8%, liver transplant=12.5% |
Hiruy et al. 2021 | Retrospective | Albumin+ midodrine+ octreotide (standardized) | 28 | Full response (SCr within 0.3mg/dl of baseline) =25%, renal replacement=21%, liver transplant=3.6%, 30-day mortality=39.2% |
Non-standardized treatment | 60 | Full response (SCr within 0.3mg/dl of baseline) = 10%, renal replacement=45%, liver transplant=23%, 30-day mortality=43% | ||
Moore et al. 2020 | Retrospective | Terlipressin+ albumin | 203 | Complete response (SCr < 1.5mg/dl) =50.2%, overall response=72.9% Renal transplant=12%, liver transplant=2%, |
Other vasoconstrictors | 22 | Complete response (SCr < 1.5mg/dl) =22.7%, overall response=59.1% | ||
Giovo et al. 2020 | Retrospective | Terlipressin+ albumin | 24 | Response to treatment=67% |
Noradrenaline+ albumin | 13 | Response to treatment=23% | ||
Nguyen-Tat et al. 2019 | Retrospective | Terlipressin + albumin for HRS-1 patients | 54 | HRS reversal=48%, relapse=8%, median OS=89±53 |
Terlipressin + albumin for HRS-2 patients | 52 | HRS reversal=46%, relapse=50%, mortality=20%, median OS=239±174 | ||
RCT= randomized clinical trial, HRS=Hepatorenal syndrome, SCr=serum creatinine, | ||||
Disclosures:
Mohammad Hamza, MD1, Jaswanth R. Jasti, MD2, Nazish Arshad, MD3, Alynna Mariz Perez, MD4, Danish Iltaf Satti, MBBS5, Wajeeha Aiman, MD6, Muhammad Yasir Anwar, MD7, Muhammad Suleman, MBBS8, Nikhilesh Nanjundaiah, MD9, Talha Azam Tarrar, MD10, Muhammad Ashar Ali, MD11. E0502 - Progress in Drug Therapy for Hepatorenal Syndrome: A Systematic Review of Clinical Studies in the Last 3 Years, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
