Back
Poster Session D - Tuesday Morning
Category: Liver
D0575 - Aloe Vera: A Boon or Bane
Tuesday, October 25, 2022
10:00 AM – 12:00 PM ET
Location: Crown Ballroom

.jpeg.jpg)
Uneza Khawaja, MD
Danbury Hospital
Danbury, CT
Presenting Author(s)
Uneza Khawaja, MD, Nida Anwaar, MD, Muhammad Masood, MD
Danbury Hospital, Danbury, CT
Introduction: Aloe Vera is derived from a cactus-like plant, a member of the Lily family that grows best in arid climates. Aloe Vera products are derived from the leaf and contain over 75 identified substances including anthraquinones, vitamins (A, C, E), enzymes, minerals, sugars, fatty acids, amino acids, salicylic acid and hormones. Aloe has been widely used in phytomedicine and is described as a herb which has anti-inflammatory, anti-proliferative, anti-aging effects.
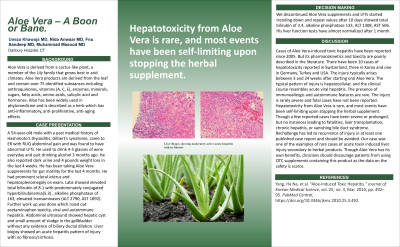
Case Description/Methods: 54-year-old male with a history of Hashimoto's thyroiditis, Gilbert's syndrome, came to ER with RUQ abdominal pain. He used to drink 4-5 glasses of wine everyday and quit 3 months ago. Also reported dark urine and a 4 pound weight loss over a month. He has been taking Aloe Vera supplements for gut motility for 4 months. Had scleral icterus and hepatosplenomegaly on exam. Labs showed elevated total bilirubin of 8.1 with direct hyperbilirubinemia(5.3) , ALP of 163, ALT 2790, AST 1892. Work up ruled out acetaminophen toxicity, viral and autoimmune hepatitis. Abdominal US showed hepatic cyst and small amount of sludge in the gallbladder without biliary ductal dilation. Liver biopsy showed an acute hepatitis pattern of injury with no fibrosis/cirrhosis. Aloe Vera supplements were discontinued and LFTs started trending down and repeat values after 10 days showed total bilirubin of 3.4, ALP 133, ALT 1308, AST 506. LFTs have almost normalized after 1 month.
Discussion: Cases of Aloe Vera-induced hepatotoxicity have been reported since 2005 but its pharmacokinetics and toxicity are poorly described in literature. It has been reported in multiple countries with the most in Switzerland. Injury typically arises between 3 and 24 weeks after starting oral Aloe Vera. Typical pattern of injury is hepatocellular and the clinical course resembles acute viral hepatitis. Injury is rarely severe and fatal cases have not been reported. Most cases of hepatotoxicity from Aloe Vera have been self-limiting upon discontinuing it. Few cases have been severe or prolonged, but no instances leading to liver transplantation, chronic hepatitis, or vanishing bile duct syndrome. Rechallenge has led to recurrence of injury in at least one published case report and should be avoided. Our case was one of the examples of rare cases of acute toxin induced liver injury secondary to herbal products. Though Aloe Vera has its benefits, patients should be discouraged from using OTC supplements containing this product as the data on the safety is scarce.
Disclosures:
Uneza Khawaja, MD, Nida Anwaar, MD, Muhammad Masood, MD. D0575 - Aloe Vera: A Boon or Bane, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
Danbury Hospital, Danbury, CT
Introduction: Aloe Vera is derived from a cactus-like plant, a member of the Lily family that grows best in arid climates. Aloe Vera products are derived from the leaf and contain over 75 identified substances including anthraquinones, vitamins (A, C, E), enzymes, minerals, sugars, fatty acids, amino acids, salicylic acid and hormones. Aloe has been widely used in phytomedicine and is described as a herb which has anti-inflammatory, anti-proliferative, anti-aging effects.
Case Description/Methods: 54-year-old male with a history of Hashimoto's thyroiditis, Gilbert's syndrome, came to ER with RUQ abdominal pain. He used to drink 4-5 glasses of wine everyday and quit 3 months ago. Also reported dark urine and a 4 pound weight loss over a month. He has been taking Aloe Vera supplements for gut motility for 4 months. Had scleral icterus and hepatosplenomegaly on exam. Labs showed elevated total bilirubin of 8.1 with direct hyperbilirubinemia(5.3) , ALP of 163, ALT 2790, AST 1892. Work up ruled out acetaminophen toxicity, viral and autoimmune hepatitis. Abdominal US showed hepatic cyst and small amount of sludge in the gallbladder without biliary ductal dilation. Liver biopsy showed an acute hepatitis pattern of injury with no fibrosis/cirrhosis. Aloe Vera supplements were discontinued and LFTs started trending down and repeat values after 10 days showed total bilirubin of 3.4, ALP 133, ALT 1308, AST 506. LFTs have almost normalized after 1 month.
Discussion: Cases of Aloe Vera-induced hepatotoxicity have been reported since 2005 but its pharmacokinetics and toxicity are poorly described in literature. It has been reported in multiple countries with the most in Switzerland. Injury typically arises between 3 and 24 weeks after starting oral Aloe Vera. Typical pattern of injury is hepatocellular and the clinical course resembles acute viral hepatitis. Injury is rarely severe and fatal cases have not been reported. Most cases of hepatotoxicity from Aloe Vera have been self-limiting upon discontinuing it. Few cases have been severe or prolonged, but no instances leading to liver transplantation, chronic hepatitis, or vanishing bile duct syndrome. Rechallenge has led to recurrence of injury in at least one published case report and should be avoided. Our case was one of the examples of rare cases of acute toxin induced liver injury secondary to herbal products. Though Aloe Vera has its benefits, patients should be discouraged from using OTC supplements containing this product as the data on the safety is scarce.
Disclosures:
Uneza Khawaja indicated no relevant financial relationships.
Nida Anwaar indicated no relevant financial relationships.
Muhammad Masood indicated no relevant financial relationships.
Uneza Khawaja, MD, Nida Anwaar, MD, Muhammad Masood, MD. D0575 - Aloe Vera: A Boon or Bane, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
