Back

Poster Session E - Tuesday Afternoon
Category: Liver
E0583 - Voxelotor (Oxbryta) Induced Liver Injury
Tuesday, October 25, 2022
3:00 PM – 5:00 PM ET
Location: Crown Ballroom

Has Audio

Ifrah Fatima, MD
University of Missouri-Kansas City
Kansas City, MO
Presenting Author(s)
Ifrah Fatima, MD1, Quinton Palmer, MD2, Tahar Mahmoudi, MD2
1University of Missouri-Kansas City, Kansas City, MO; 2University of Missouri, Kansas City, MO
Introduction: Voxelotor is a HbS polymerization inhibitor approved by FDA in 2019 for treatment of sickle cell disease. Drug-induced liver injury (DILI) is one of the most common causes of acute liver injury and accounts for approximately 10% of all acute hepatitis. We present a rare case of DILI associated with Voxelotor at the dose of 1500mg which has not been previously reported.
Case Description/Methods: A 37-year-old male with a past medical history of HbSC disease was on Hydroxyurea 1.5g for 2 years which was discontinued due to pancytopenia. He was started on Voxelotor 1500mg daily with improvement in sickle cell symptoms, indirect bilirubin, hemoglobin, and reticulocyte count. He tolerated Voxelotor well. He had elevated alanine transaminase (ALT) 47 U/L after 4 months of therapy. Aspartate transaminase (AST) and ALT were uptrending at monthly follow-ups. Liver enzymes peaked at 6 months with AST 90 U/L and ALT 95 U/L. Alkaline phosphatase and total bilirubin were normal. Voxelotor 1500 mg daily was held for a week with improvement in liver enzymes- AST 35 U/L and ALT 45 U/L.
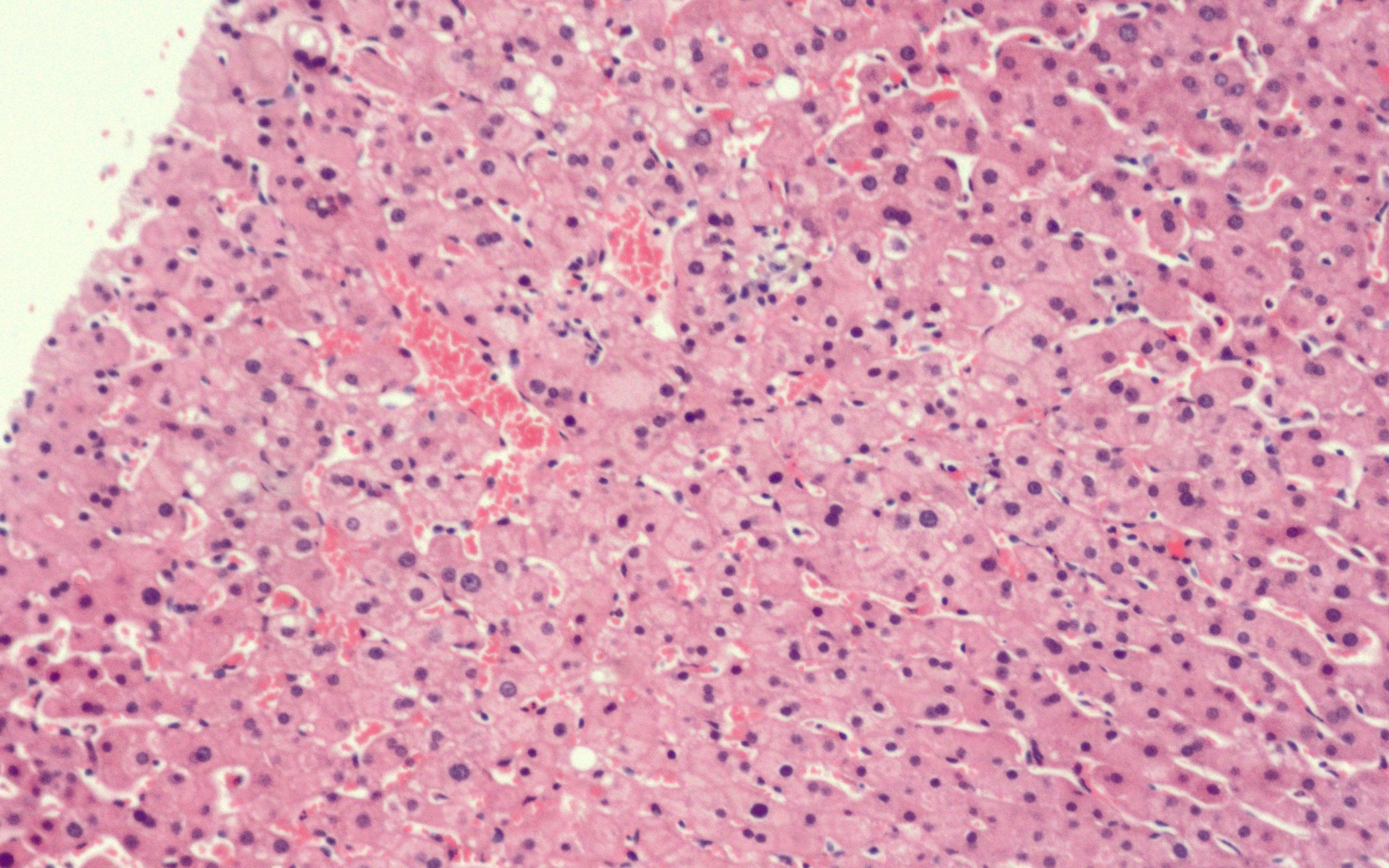
A thorough review of history and physical examination was performed. Patient was asymptomatic. BMI was 23.8 kg/m2 and he drank alcohol socially. He was not on any other hepatotoxic, over-the-counter, or herbal medications. Acute hepatitis panel was negative. The R factor was 3.6, indicating a mixed hepatocellular-cholestatic pattern. Chronic liver disease workup including ceruloplasmin, A1AT, IgG, LKMA, AMA, ASMA were negative. Fibroscan showed no fibrosis or steatosis (F0S0). US gallbladder noted cholelithiasis with normal bile ducts. Liver biopsy showed mild sinusoidal congestion, normal architecture, no steatosis, hepatitis, or necrosis (Fig 1). Given the benefits of Voxelotor for sickle cell disease and negative workup, it was restarted at a lower dose of 1g. We recommended monitoring liver enzymes every 3-6 months and repeating Fibroscan in 1 year.
Discussion: DILI can be diagnosed based on correlation with exposure and improvement with cessation of drug. Only 2.2% of patients were reported to have elevated AST with a dose of 900mg in the Voxelotor randomized clinical trial. However, liver injury with Voxelotor at the dose of 1500mg was not reported. We described the first reported case of DILI with Voxelotor 1500 mg daily. Providers should be aware of this potential DILI and consider the risk and benefits of withdrawing treatment.
Disclosures:
Ifrah Fatima, MD1, Quinton Palmer, MD2, Tahar Mahmoudi, MD2. E0583 - Voxelotor (Oxbryta) Induced Liver Injury, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1University of Missouri-Kansas City, Kansas City, MO; 2University of Missouri, Kansas City, MO
Introduction: Voxelotor is a HbS polymerization inhibitor approved by FDA in 2019 for treatment of sickle cell disease. Drug-induced liver injury (DILI) is one of the most common causes of acute liver injury and accounts for approximately 10% of all acute hepatitis. We present a rare case of DILI associated with Voxelotor at the dose of 1500mg which has not been previously reported.
Case Description/Methods: A 37-year-old male with a past medical history of HbSC disease was on Hydroxyurea 1.5g for 2 years which was discontinued due to pancytopenia. He was started on Voxelotor 1500mg daily with improvement in sickle cell symptoms, indirect bilirubin, hemoglobin, and reticulocyte count. He tolerated Voxelotor well. He had elevated alanine transaminase (ALT) 47 U/L after 4 months of therapy. Aspartate transaminase (AST) and ALT were uptrending at monthly follow-ups. Liver enzymes peaked at 6 months with AST 90 U/L and ALT 95 U/L. Alkaline phosphatase and total bilirubin were normal. Voxelotor 1500 mg daily was held for a week with improvement in liver enzymes- AST 35 U/L and ALT 45 U/L.
A thorough review of history and physical examination was performed. Patient was asymptomatic. BMI was 23.8 kg/m2 and he drank alcohol socially. He was not on any other hepatotoxic, over-the-counter, or herbal medications. Acute hepatitis panel was negative. The R factor was 3.6, indicating a mixed hepatocellular-cholestatic pattern. Chronic liver disease workup including ceruloplasmin, A1AT, IgG, LKMA, AMA, ASMA were negative. Fibroscan showed no fibrosis or steatosis (F0S0). US gallbladder noted cholelithiasis with normal bile ducts. Liver biopsy showed mild sinusoidal congestion, normal architecture, no steatosis, hepatitis, or necrosis (Fig 1). Given the benefits of Voxelotor for sickle cell disease and negative workup, it was restarted at a lower dose of 1g. We recommended monitoring liver enzymes every 3-6 months and repeating Fibroscan in 1 year.
Discussion: DILI can be diagnosed based on correlation with exposure and improvement with cessation of drug. Only 2.2% of patients were reported to have elevated AST with a dose of 900mg in the Voxelotor randomized clinical trial. However, liver injury with Voxelotor at the dose of 1500mg was not reported. We described the first reported case of DILI with Voxelotor 1500 mg daily. Providers should be aware of this potential DILI and consider the risk and benefits of withdrawing treatment.

Figure: Liver biopsy showing mild sinusoidal congestion
Disclosures:
Ifrah Fatima indicated no relevant financial relationships.
Quinton Palmer indicated no relevant financial relationships.
Tahar Mahmoudi indicated no relevant financial relationships.
Ifrah Fatima, MD1, Quinton Palmer, MD2, Tahar Mahmoudi, MD2. E0583 - Voxelotor (Oxbryta) Induced Liver Injury, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
