Back


Poster Session C - Monday Afternoon
Category: Liver
C0552 - A Superfood With a Dark Side: A Case of Severe Liver Injury Due to Turmeric
Monday, October 24, 2022
3:00 PM – 5:00 PM ET
Location: Crown Ballroom

Has Audio

Angeline Luong, MD
Kaiser Permanente Loe Angeles Medical Center
Pasadena, CA
Presenting Author(s)
Award: ACG Case Reports Journal Award (Trainee)
Award: Presidential Poster Award
Angeline Liu, MD1, Paul Chang, MD2
1Kaiser Permanente Loe Angeles Medical Center, Pasadena, CA; 2Kaiser Permanente Los Angeles Medical Center, Los Angeles, CA
Introduction: Herbal and dietary supplements are a common cause of drug induced liver injury. Turmeric is a supplement used for anti-inflammatory and anti-neoplastic effects believed to have a good safety profile. Turmeric induced severe liver injury is a rare entity that has not been well-described.
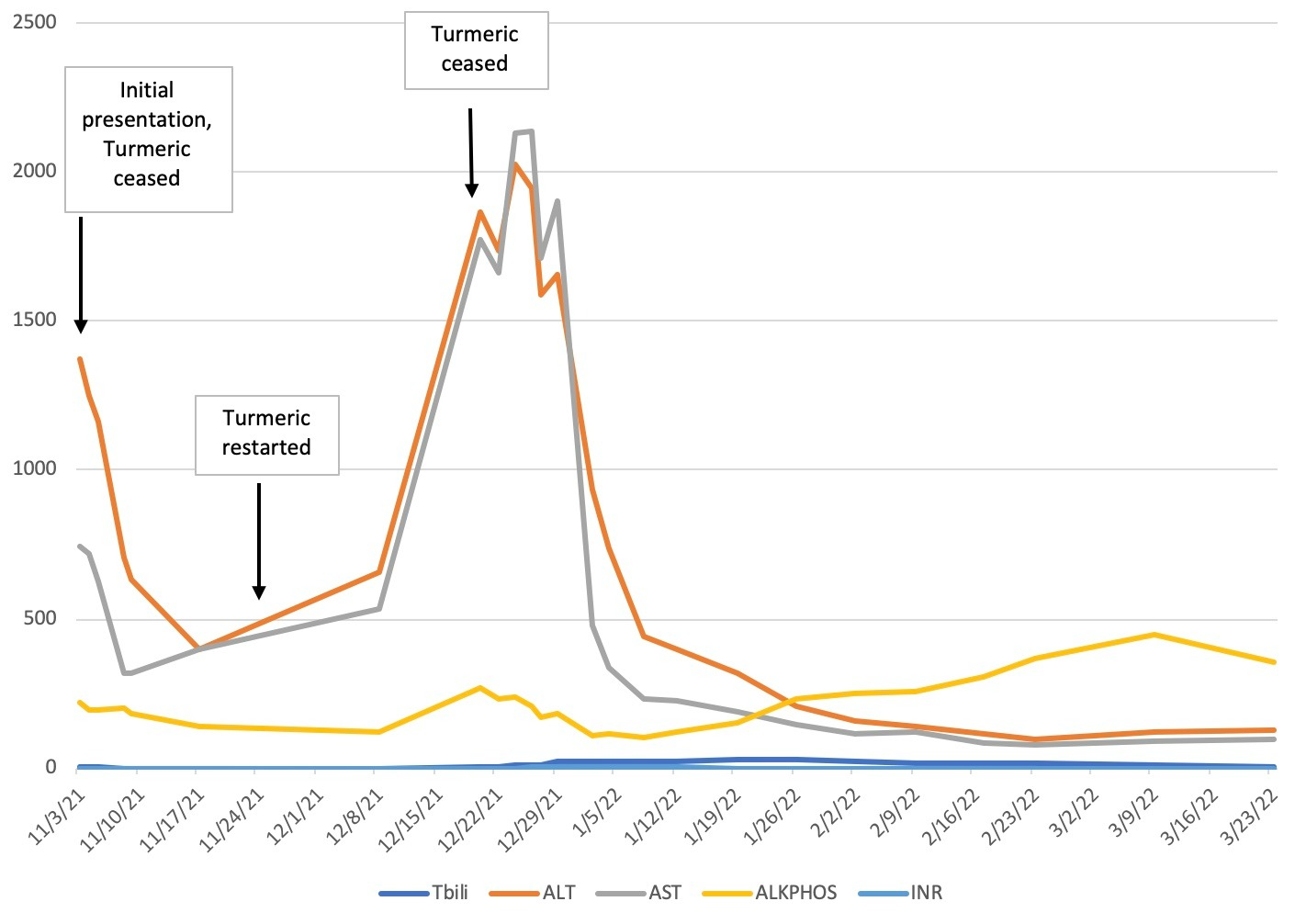
Case Description/Methods: A 49 year old female with no significant medical history was admitted for acute liver injury with total bilirubin 2.0, alanine aminotransferase (ALT) 1300, aspartate aminotransferase (AST) 745, alkaline phosphatase (ALP) 220, international normalized ratio (INR) 1.1. Workup of her liver injury with serologic workup and imaging were negative. She admitted to taking a 1000 milligram daily dose of turmeric in formulation with black pepper for the past 3 months. Her liver tests improved and she was discharged with instructions to avoid further herbal supplements. Two weeks later, outpatient labs showed improving liver tests with total bilirubin 0.9, ALT 397, AST 320, ALP 138, INR 1.0
One month later, she was readmitted and was found to have total bilirubin 3.9, ALT 1865, AST 1770, ALP 269, INR 1.2. Repeated serologic workup and imaging were again unremarkable. Her liver tests continued to increase, peaking with total bilirubin 27.8, ALT and AST above 2000, INR 2.3 without encephalopathy. Percutaneous liver biopsy showed severe acute hepatitis with hepatocyte dropout and focal parenchymal collapse without significant fibrosis, concerning for drug-induced liver injury. She admitted that a few weeks after her first hospitalization, she resumed taking turmeric.
Due to rising INR, she was treated with prednisone and monitored closely. After an extended hospitalization, she was discharged home with a prednisone taper. On discharge, total bilirubin was 23, ALT 445, AST 231, ALP 124, INR 1.6. Over the next 6 months, her liver tests significantly improved, including normalization of bilirubin and INR. She continues to avoid turmeric.
Discussion: While studies have reported mild liver function test abnormalities with turmeric use, this case illustrates a rare example of severe turmeric induced liver injury in the setting of a positive rechallenge. The risk of severe liver injury may be increased when turmeric is taken in formulation with black pepper, which aids intestinal absorption of turmeric’s active ingredient curcumin. As turmeric is a highly unregulated supplement, physicians should be aware of patients who are taking this supplement and the potential adverse effects associated with it.
Disclosures:
Angeline Liu, MD1, Paul Chang, MD2. C0552 - A Superfood With a Dark Side: A Case of Severe Liver Injury Due to Turmeric, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
Award: Presidential Poster Award
Angeline Liu, MD1, Paul Chang, MD2
1Kaiser Permanente Loe Angeles Medical Center, Pasadena, CA; 2Kaiser Permanente Los Angeles Medical Center, Los Angeles, CA
Introduction: Herbal and dietary supplements are a common cause of drug induced liver injury. Turmeric is a supplement used for anti-inflammatory and anti-neoplastic effects believed to have a good safety profile. Turmeric induced severe liver injury is a rare entity that has not been well-described.
Case Description/Methods: A 49 year old female with no significant medical history was admitted for acute liver injury with total bilirubin 2.0, alanine aminotransferase (ALT) 1300, aspartate aminotransferase (AST) 745, alkaline phosphatase (ALP) 220, international normalized ratio (INR) 1.1. Workup of her liver injury with serologic workup and imaging were negative. She admitted to taking a 1000 milligram daily dose of turmeric in formulation with black pepper for the past 3 months. Her liver tests improved and she was discharged with instructions to avoid further herbal supplements. Two weeks later, outpatient labs showed improving liver tests with total bilirubin 0.9, ALT 397, AST 320, ALP 138, INR 1.0
One month later, she was readmitted and was found to have total bilirubin 3.9, ALT 1865, AST 1770, ALP 269, INR 1.2. Repeated serologic workup and imaging were again unremarkable. Her liver tests continued to increase, peaking with total bilirubin 27.8, ALT and AST above 2000, INR 2.3 without encephalopathy. Percutaneous liver biopsy showed severe acute hepatitis with hepatocyte dropout and focal parenchymal collapse without significant fibrosis, concerning for drug-induced liver injury. She admitted that a few weeks after her first hospitalization, she resumed taking turmeric.
Due to rising INR, she was treated with prednisone and monitored closely. After an extended hospitalization, she was discharged home with a prednisone taper. On discharge, total bilirubin was 23, ALT 445, AST 231, ALP 124, INR 1.6. Over the next 6 months, her liver tests significantly improved, including normalization of bilirubin and INR. She continues to avoid turmeric.
Discussion: While studies have reported mild liver function test abnormalities with turmeric use, this case illustrates a rare example of severe turmeric induced liver injury in the setting of a positive rechallenge. The risk of severe liver injury may be increased when turmeric is taken in formulation with black pepper, which aids intestinal absorption of turmeric’s active ingredient curcumin. As turmeric is a highly unregulated supplement, physicians should be aware of patients who are taking this supplement and the potential adverse effects associated with it.

Figure: Liver tests over time in the setting of turmeric cessation and rechallenge.
Disclosures:
Angeline Liu indicated no relevant financial relationships.
Paul Chang indicated no relevant financial relationships.
Angeline Liu, MD1, Paul Chang, MD2. C0552 - A Superfood With a Dark Side: A Case of Severe Liver Injury Due to Turmeric, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.


