Back
Poster Session E - Tuesday Afternoon
Category: Practice Management
E0616 - The Implementation of Multidrug-Resistant Bacterial Testing to Prioritize Duodenoscope Sterilization: Experience From a High-Volume Health System
Tuesday, October 25, 2022
3:00 PM – 5:00 PM ET
Location: Crown Ballroom


Jad AbiMansour, MD
Mayo Clinic
Rochester, MN
Presenting Author(s)
Jad AbiMansour, MD, Eric J. Vargas, MD, MS, John A. Martin, MD, Bret T Petersen, MD
Mayo Clinic, Rochester, MN
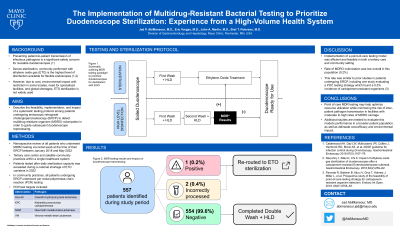
Introduction: Preventing patient-to-patient transmission of infectious pathogens is a significant safety concern for reusable duodenoscopes. Device sterilization, commonly performed with ethylene oxide gas (ETO) is the highest level of disinfection available for flexible endoscopes. However, due to cost, environmental impact with restriction in some locales, need for specialized facilities, and global shortages, ETO sterilization is not widely used. The aim of this study is to describe the feasibility, implementation, and impact of a systematic testing protocol among patients undergoing endoscopic retrograde cholangiopancreatoscopy (ERCP) to detect multidrug resistant organism (MDRO) colonization as a guide to subsequent duodenoscope reprocessing.
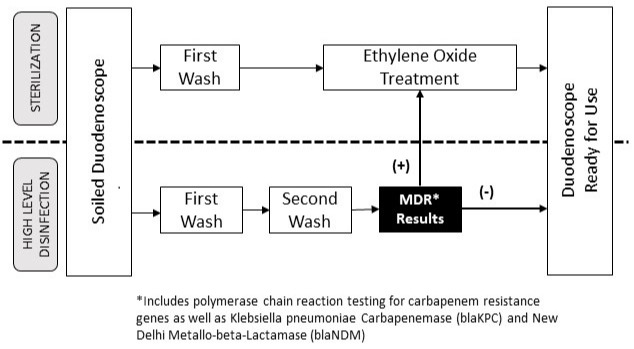
Methods: We performed a retrospective review of all patients who underwent MDRO testing via rectal swab at the time of their ERCP between January 2018 and May 2022 at a tertiary care center and satellite community practices within a single healthcare system. Per rectal polymerase chain reaction (PCR) testing was performed in all patients in two community practices throughout 2018 to the present and after daily sterilization capacity was exceeded in the tertiary center during a national shortage of ETO canisters in 2022 (Figure 1). PCR test targets included oxacillin-hydrolyzing beta-lactamase (Oxa-48), Klebsiella pneumoniae carbapenemase (KPC), New Delhi metallo-beta-Lactamase (NDM), and Verona metallo-beta-Lactamase (VIM).
Results: A total of 557 patients underwent testing. Of these, only 1 (0.2%) returned positive. There were also 2 (0.4%) tests which were processed incorrectly, prompting precautionary diversion to sterilization with ETO. All duodenoscopes used in patients with negative PCR tests were reprocessed with double washing and high-level disinfection (DHLD). No patients from the study period were reported to have developed a healthcare associated infection or MDRO-related disease.
Discussion: The rate of MDRO colonization was low in this study population. However, the implementation of a point-of-care testing model was efficient, feasible, and may help optimize resource utilization while minimizing the risk of inter-patient pathogen transmission in facilities with moderate to high rates of MDRO carriage. Additional studies are needed to evaluate this model’s performance in a broader patient population, as well as delineate cost-efficacy and environmental impact.
Disclosures:
Jad AbiMansour, MD, Eric J. Vargas, MD, MS, John A. Martin, MD, Bret T Petersen, MD. E0616 - The Implementation of Multidrug-Resistant Bacterial Testing to Prioritize Duodenoscope Sterilization: Experience From a High-Volume Health System, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
Mayo Clinic, Rochester, MN
Introduction: Preventing patient-to-patient transmission of infectious pathogens is a significant safety concern for reusable duodenoscopes. Device sterilization, commonly performed with ethylene oxide gas (ETO) is the highest level of disinfection available for flexible endoscopes. However, due to cost, environmental impact with restriction in some locales, need for specialized facilities, and global shortages, ETO sterilization is not widely used. The aim of this study is to describe the feasibility, implementation, and impact of a systematic testing protocol among patients undergoing endoscopic retrograde cholangiopancreatoscopy (ERCP) to detect multidrug resistant organism (MDRO) colonization as a guide to subsequent duodenoscope reprocessing.
Methods: We performed a retrospective review of all patients who underwent MDRO testing via rectal swab at the time of their ERCP between January 2018 and May 2022 at a tertiary care center and satellite community practices within a single healthcare system. Per rectal polymerase chain reaction (PCR) testing was performed in all patients in two community practices throughout 2018 to the present and after daily sterilization capacity was exceeded in the tertiary center during a national shortage of ETO canisters in 2022 (Figure 1). PCR test targets included oxacillin-hydrolyzing beta-lactamase (Oxa-48), Klebsiella pneumoniae carbapenemase (KPC), New Delhi metallo-beta-Lactamase (NDM), and Verona metallo-beta-Lactamase (VIM).
Results: A total of 557 patients underwent testing. Of these, only 1 (0.2%) returned positive. There were also 2 (0.4%) tests which were processed incorrectly, prompting precautionary diversion to sterilization with ETO. All duodenoscopes used in patients with negative PCR tests were reprocessed with double washing and high-level disinfection (DHLD). No patients from the study period were reported to have developed a healthcare associated infection or MDRO-related disease.
Discussion: The rate of MDRO colonization was low in this study population. However, the implementation of a point-of-care testing model was efficient, feasible, and may help optimize resource utilization while minimizing the risk of inter-patient pathogen transmission in facilities with moderate to high rates of MDRO carriage. Additional studies are needed to evaluate this model’s performance in a broader patient population, as well as delineate cost-efficacy and environmental impact.

Figure: Figure 1. Workflow utilizing multidrug resistance gene testing to prioritize duodenoscopes for sterilization with ethylene oxide gas.
Disclosures:
Jad AbiMansour indicated no relevant financial relationships.
Eric Vargas indicated no relevant financial relationships.
John Martin indicated no relevant financial relationships.
Bret T Petersen: AMBU – Grant/Research Support. Boston scientific – Grant/Research Support. Olympus – Consultant.
Jad AbiMansour, MD, Eric J. Vargas, MD, MS, John A. Martin, MD, Bret T Petersen, MD. E0616 - The Implementation of Multidrug-Resistant Bacterial Testing to Prioritize Duodenoscope Sterilization: Experience From a High-Volume Health System, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
