Back

Poster Session E - Tuesday Afternoon
Category: IBD
E0410 - Induction of Inflammatory Bowel Disease by Interleukin-17 Inhibitor
Tuesday, October 25, 2022
3:00 PM – 5:00 PM ET
Location: Crown Ballroom

Has Audio
- JT
Jin Tao, MS, MD
MercyHealth
Rockford, IL
Presenting Author(s)
Jin Tao, MS, MD1, Luqman Baloch, MD2, Altaf Dawood, MD2, Naser Khan, MD1
1MercyHealth, Rockford, IL; 2MercyHealth Internal Medicine Residency Program, Rockford, IL
Introduction: Ixekizumab is a monoclonal antibody targeting interleukin-17 (IL-17) approved for treating psoriasis. We report a case with rare gastrointestinal (GI) manifestation with ixekizumab use.
Case Description/Methods: A 40-year-old male with psoriasis presented with 4 weeks of abdominal pain and intermittent watery diarrhea with blood & mucous. He was febrile to 102 °F with other vitals unremarkable. Physical exam revealed psoriatic plaques, generalized abdominal tenderness, & bilateral knee stiffness. Patient’s medications included topical betamethasone-calcipotriene, methotrexate weekly, folic acid, golimumab monthly, and piroxicam. Two months prior, he began taking ixekizumab for worsening psoriasis. Psoriatic lesions and arthropathy improved. However, hematochezia started one month after using ixekizumab.
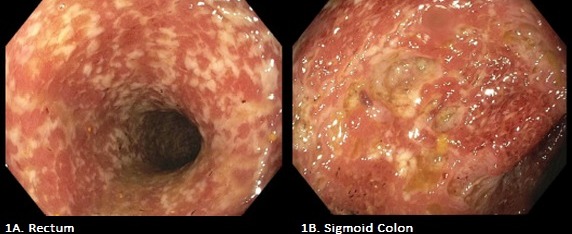
Work up revealed WBC 15.1k/mm3, CRP 174.4 mg/L, and ESR 76 mm/hr. CT abdomen and pelvis with IV and oral contrast demonstrated diffuse circumferential long segment wall thickening of the distal colon and rectum with mucosal enhancement. Patient continued to report daily hematochezia. Colonoscopy demonstrated a contiguous area of bleeding ulcerated mucosa in his rectum, sigmoid colon, descending colon and splenic flexure (Figure 1). Biopsies demonstrated crypt architecture abnormalities including bifurcation of crypts, prominent chronic inflammation consisting of lymphocytes, plasma cells, and eosinophils with mucosal ulcerations and crypt abscess formation. No granulomas were noted. Stains for HSV & CMV, and GI pathogen panel were negative. This patient’s clinical findings were most consistent with ulcerative colitis (UC). Ixekizumab was discontinued. Hydrocortisone and mesalamine were started. Shortly thereafter all GI symptoms completely resolved and patient was discharged home.
Discussion: Here we present a patient with history of psoriasis, who developed severe UC in the context of recent IL-17 inhibitor use. Clinical trials investigating IL-17 inhibition in inflammatory bowel disease (IBD) suggest that it may cause worsening or relapse in symptoms. We present a unique case of ixekizumab causing new-onset UC. This patient had no family history of IBD, no smoking history, & no extra-intestinal manifestations suggestive of UC. Only after introduction of ixekizumab he developed symptoms and pathology related to UC.
This case describes rare GI manifestations of ixekizumab and other IL-17 inhibitors. It reminds us to be cognizant and monitor for IBD symptoms in patients taking such medications.
Disclosures:
Jin Tao, MS, MD1, Luqman Baloch, MD2, Altaf Dawood, MD2, Naser Khan, MD1. E0410 - Induction of Inflammatory Bowel Disease by Interleukin-17 Inhibitor, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1MercyHealth, Rockford, IL; 2MercyHealth Internal Medicine Residency Program, Rockford, IL
Introduction: Ixekizumab is a monoclonal antibody targeting interleukin-17 (IL-17) approved for treating psoriasis. We report a case with rare gastrointestinal (GI) manifestation with ixekizumab use.
Case Description/Methods: A 40-year-old male with psoriasis presented with 4 weeks of abdominal pain and intermittent watery diarrhea with blood & mucous. He was febrile to 102 °F with other vitals unremarkable. Physical exam revealed psoriatic plaques, generalized abdominal tenderness, & bilateral knee stiffness. Patient’s medications included topical betamethasone-calcipotriene, methotrexate weekly, folic acid, golimumab monthly, and piroxicam. Two months prior, he began taking ixekizumab for worsening psoriasis. Psoriatic lesions and arthropathy improved. However, hematochezia started one month after using ixekizumab.
Work up revealed WBC 15.1k/mm3, CRP 174.4 mg/L, and ESR 76 mm/hr. CT abdomen and pelvis with IV and oral contrast demonstrated diffuse circumferential long segment wall thickening of the distal colon and rectum with mucosal enhancement. Patient continued to report daily hematochezia. Colonoscopy demonstrated a contiguous area of bleeding ulcerated mucosa in his rectum, sigmoid colon, descending colon and splenic flexure (Figure 1). Biopsies demonstrated crypt architecture abnormalities including bifurcation of crypts, prominent chronic inflammation consisting of lymphocytes, plasma cells, and eosinophils with mucosal ulcerations and crypt abscess formation. No granulomas were noted. Stains for HSV & CMV, and GI pathogen panel were negative. This patient’s clinical findings were most consistent with ulcerative colitis (UC). Ixekizumab was discontinued. Hydrocortisone and mesalamine were started. Shortly thereafter all GI symptoms completely resolved and patient was discharged home.
Discussion: Here we present a patient with history of psoriasis, who developed severe UC in the context of recent IL-17 inhibitor use. Clinical trials investigating IL-17 inhibition in inflammatory bowel disease (IBD) suggest that it may cause worsening or relapse in symptoms. We present a unique case of ixekizumab causing new-onset UC. This patient had no family history of IBD, no smoking history, & no extra-intestinal manifestations suggestive of UC. Only after introduction of ixekizumab he developed symptoms and pathology related to UC.
This case describes rare GI manifestations of ixekizumab and other IL-17 inhibitors. It reminds us to be cognizant and monitor for IBD symptoms in patients taking such medications.

Figure: Figure 1: Colonoscopy demonstrated a contiguous area of bleeding ulcerated mucosa in the rectum (1A) and sigmoid colon (1B).
Disclosures:
Jin Tao indicated no relevant financial relationships.
Luqman Baloch indicated no relevant financial relationships.
Altaf Dawood indicated no relevant financial relationships.
Naser Khan indicated no relevant financial relationships.
Jin Tao, MS, MD1, Luqman Baloch, MD2, Altaf Dawood, MD2, Naser Khan, MD1. E0410 - Induction of Inflammatory Bowel Disease by Interleukin-17 Inhibitor, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
