Poster Session D - Tuesday Morning
Category: IBD
D0371 - Non-Biologic Medication Use Pre- and Post-Ustekinumab Initiation Among Patients With Ulcerative Colitis

- SK
Sumesh Kachroo, PhD
Janssen Scientific Affairs, LLC
Horsham, Pennsylvania
Presenting Author(s)
1Analysis Group, Inc., Montreal, PQ, Canada; 2Janssen Scientific Affairs, LLC, Horsham, PA
Introduction: Among patients with ulcerative colitis (UC), treatment with immunomodulators, 5-ASA (5-aminosalicylic acid), and corticosteroids while receiving biologic agents is common. However, real-world information on non-biologic medication use among UC patients initiated on ustekinumab is limited. This study compared non-biologic medication use pre- and post-ustekinumab initiation among UC patients.
Methods: Adults with UC initiated on ustekinumab (index date) between 10/18/2019 (FDA approval of ustekinumab for UC) and 10/31/2020 were selected from the de-identified health insurance claims Symphony Health Solutions’ Patient Transactional Datasets. Patients were excluded if they had a claim for Crohn’s disease anytime or for other autoimmune diseases pre-index. Patients were required to have: ≥1 claim for UC in the 12 months pre-index period (baseline), ≥2 ustekinumab claims within 90 days of the index date, and ≥6 months of follow-up. Non-biologic agent use in the 6 months period before and after the initiation of ustekinumab was compared with a logistic model estimated by generalized estimating equation. Results were reported as odds ratio with 95% confidence interval and p-values.
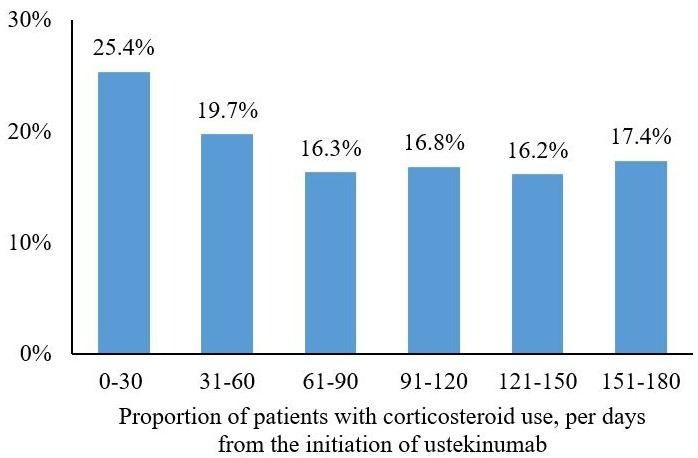
Results: A total of 760 patients with UC were initiated on ustekinumab and selected for the study (age 44.6 years old; 48.9% female; baseline biologic use 52.1% [potentially underestimated given the database open nature]; Quan-Charlson comorbidity index 0.62). The likelihood of immunomodulator and 5-ASA use decreased by 22% and 46%, after ustekinumab initiation (all P< 0.05). Similarly, patients were 52% less likely to use any corticosteroids and 34% less likely to use corticosteroids for ≥60 days (all P< 0.05). Further, there was no significant changes observed for the use of opioids (18.9% vs. 17.4%) or the use of anti-diarrheals (5.9% vs. 6.1%), and patients were 29% less likely to use GI antispasmodics after ustekinumab initiation (P< 0.05; Table 1). In a descriptive analysis, corticosteroids use numerically decreased at each month post-ustekinumab from 25.4% during the first 30 days following the index date compared to 17.4% within 150-180 days post-ustekinumab (Figure 1).
Discussion: In this real-world study of patients with UC, initiating ustekinumab was associated with a significant decrease in the use of immunomodulators, 5-ASA, and corticosteroids. Longer-term data are necessary to better understand non-biologic medication use after biologic initiation and inform treatment choice for patients with UC.
| Pre-ustekinumab | Post-ustekinumab | Odds ratio1 (95% CI), |
| N = 760 | ||
Use of immunomodulators | 120 (15.8) | 97 (12.8) | 0.78 (0.64, 0.95), 0.014* |
Use of 5-ASA | 312 (41.1) | 208 (27.4) | 0.54 (0.47, 0.62), < 0.001* |
Use of corticosteroids | 481 (63.3) | 346 (45.5) | 0.48 (0.41, 0.57), < 0.001* |
Cumulative use2 ≥60 days | 243 (32.0) | 180 (23.7) | 0.66 (0.55, 0.79), < 0.001* |
Cumulative use2 ≥90 days | 151 (19.9) | 114 (15.0) | 0.71 (0.58, 0.87), 0.001* |
≥1 episode3 ≥ 60 days | 201 (26.4) | 156 (20.5) | 0.72 (0.59, 0.87), < 0.001* |
≥1 episode3 ≥ 90 days | 118 (15.5) | 89 (11.7) | 0.72 (0.56, 0.93), 0.013* |
Use of opioids | 144 (18.9) | 132 (17.4) | 0.90 (0.74, 1.10), 0.296 |
Use of antidiarrheals | 45 (5.9) | 46 (6.1) | 1.02 (0.76, 1.38), 0.879 |
Use of GI antispasmodics | 86 (11.3) | 63 (8.3) | 0.71 (0.54, 0.93), 0.013* |
Abbreviations: CI: Confidence interval; GI: Gastrointestinal; 5-ASA: 5-aminosalicylic acid
Notes: (1) Odds ratio obtained from a logistic model estimated with generalized estimating equation;
(2) Non-overlapping days of supply included; (3) A therapy exposure gap of 14 days of supply was used
to define continuous use
Disclosures:
Dominic Pilon, MA1, Ruizhi Zhao, PhD2, Ameur M. Manceur, PhD1, Zhijie Ding, PhD, MS2, Sumesh Kachroo, PhD2, Maude Vermette-Laforme, BSc1, Patrick Lefebvre, MA1. D0371 - Non-Biologic Medication Use Pre- and Post-Ustekinumab Initiation Among Patients With Ulcerative Colitis, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
