Back

Poster Session D - Tuesday Morning
Category: IBD
D0377 - Long-Term Outcomes of Treatment With and De-Escalation From a Combination of Vedolizumab and Another Biologic or Tofacitinib for Inflammatory Bowel Disease
Tuesday, October 25, 2022
10:00 AM – 12:00 PM ET
Location: Crown Ballroom

Has Audio

Jenny Jan, MD
UT Southwestern
Dallas, TX
Presenting Author(s)
Jenny Jan, MD1, David I. Fudman, MD2, Ernesto M. Llano, MD2, Shreeju Shrestha, MSN2, Ezra Burstein, MD, PhD2, Moheb Boktor, MD2
1UT Southwestern, Dallas, TX; 2University of Texas Southwestern, Dallas, TX
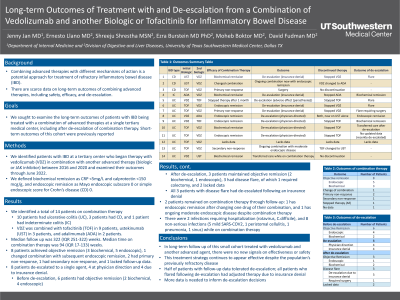
Introduction: There are scant data on long-term outcomes of treatment of inflammatory bowel disease (IBD) with a combination of advanced therapies, including after de-escalation.
Methods: We identified patients with IBD at a tertiary center who began therapy with vedolizumab (VDZ) in combination with another advanced therapy (biologic or JAK inhibitor) between 2016 and 2020 and examined their outcomes through 6/1/22. We defined biochemical remission as CRP< 5 mg/L and calprotectin < 150 mcg/g, and endoscopic remission as Mayo endoscopic subscore 0 or simple endoscopic score for Crohn’s disease (CD) 0. Short-term outcomes of this cohort were previously reported.
Results: Fourteen patients with a median of 322 (IQR 251-322) weeks of follow up were identified. 10 had ulcerative colitis, 3 CD, and 1 indeterminate colitis. VDZ was combined with tofacitinib in 9 patients, ustekinumab in 3 and adalimumab in 2. Median time on combination therapy was 94 weeks (IQR 17-133). Eight patients achieved objective remission (3 biochemical, 5 endoscopic), 1 changed combination with subsequent endoscopic remission, 2 had primary non-response, 1 had secondary non-response, 1 stopped within 1 month due to reported adverse effect (paresthesia), and 1 lacked follow-up data. Eight patients de-escalated to a single agent, 4 at physician direction and 4 due to insurance denial. Before de-escalation, 6 had objective remission (2 biochemical, 4 endoscopic). After de-escalation, 3 patients maintained objective remission (2 biochemical, 1 endoscopic), 3 had disease flare, of which 1 required colectomy, and 2 lacked data. All 3 patients with disease flare had de-escalated following an insurance denial. Two patients remained on combination therapy through follow up: 1 has endoscopic remission after changing one drug of their combination and 1 has ongoing moderate endoscopic disease despite combination therapy. There were 2 infections requiring hospitalization (rotavirus, C. difficile), and 8 non-serious infections (5 mild SARS-COV-2, 1 peristomal cellulitis, 1 pneumonia, 1 sinus) while on combination therapy.
Discussion: In long-term follow up of this small cohort, there were no new signals on effectiveness or safety of combining advanced agents. De-escalation to a single agent was tolerated in half of patients with follow-up data; all patients who flared following de-escalation had adjusted therapy due to insurance denial. More data is needed to inform de-escalation decisions.
Disclosures:
Jenny Jan, MD1, David I. Fudman, MD2, Ernesto M. Llano, MD2, Shreeju Shrestha, MSN2, Ezra Burstein, MD, PhD2, Moheb Boktor, MD2. D0377 - Long-Term Outcomes of Treatment With and De-Escalation From a Combination of Vedolizumab and Another Biologic or Tofacitinib for Inflammatory Bowel Disease, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1UT Southwestern, Dallas, TX; 2University of Texas Southwestern, Dallas, TX
Introduction: There are scant data on long-term outcomes of treatment of inflammatory bowel disease (IBD) with a combination of advanced therapies, including after de-escalation.
Methods: We identified patients with IBD at a tertiary center who began therapy with vedolizumab (VDZ) in combination with another advanced therapy (biologic or JAK inhibitor) between 2016 and 2020 and examined their outcomes through 6/1/22. We defined biochemical remission as CRP< 5 mg/L and calprotectin < 150 mcg/g, and endoscopic remission as Mayo endoscopic subscore 0 or simple endoscopic score for Crohn’s disease (CD) 0. Short-term outcomes of this cohort were previously reported.
Results: Fourteen patients with a median of 322 (IQR 251-322) weeks of follow up were identified. 10 had ulcerative colitis, 3 CD, and 1 indeterminate colitis. VDZ was combined with tofacitinib in 9 patients, ustekinumab in 3 and adalimumab in 2. Median time on combination therapy was 94 weeks (IQR 17-133). Eight patients achieved objective remission (3 biochemical, 5 endoscopic), 1 changed combination with subsequent endoscopic remission, 2 had primary non-response, 1 had secondary non-response, 1 stopped within 1 month due to reported adverse effect (paresthesia), and 1 lacked follow-up data. Eight patients de-escalated to a single agent, 4 at physician direction and 4 due to insurance denial. Before de-escalation, 6 had objective remission (2 biochemical, 4 endoscopic). After de-escalation, 3 patients maintained objective remission (2 biochemical, 1 endoscopic), 3 had disease flare, of which 1 required colectomy, and 2 lacked data. All 3 patients with disease flare had de-escalated following an insurance denial. Two patients remained on combination therapy through follow up: 1 has endoscopic remission after changing one drug of their combination and 1 has ongoing moderate endoscopic disease despite combination therapy. There were 2 infections requiring hospitalization (rotavirus, C. difficile), and 8 non-serious infections (5 mild SARS-COV-2, 1 peristomal cellulitis, 1 pneumonia, 1 sinus) while on combination therapy.
Discussion: In long-term follow up of this small cohort, there were no new signals on effectiveness or safety of combining advanced agents. De-escalation to a single agent was tolerated in half of patients with follow-up data; all patients who flared following de-escalation had adjusted therapy due to insurance denial. More data is needed to inform de-escalation decisions.
Disclosures:
Jenny Jan indicated no relevant financial relationships.
David Fudman: Pfizer – Advisory Committee/Board Member.
Ernesto Llano indicated no relevant financial relationships.
Shreeju Shrestha indicated no relevant financial relationships.
Ezra Burstein: Alimentiv – Data and Safety Monitoring Board.
Moheb Boktor indicated no relevant financial relationships.
Jenny Jan, MD1, David I. Fudman, MD2, Ernesto M. Llano, MD2, Shreeju Shrestha, MSN2, Ezra Burstein, MD, PhD2, Moheb Boktor, MD2. D0377 - Long-Term Outcomes of Treatment With and De-Escalation From a Combination of Vedolizumab and Another Biologic or Tofacitinib for Inflammatory Bowel Disease, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
