Back

Poster Session C - Monday Afternoon
Category: General Endoscopy
C0291 - ColoWrap Real-World Evidence: Colonoscopy Compression Device Mitigates Ergonomic Hazards for Endoscopists and Staff
Monday, October 24, 2022
3:00 PM – 5:00 PM ET
Location: Crown Ballroom

Has Audio

Seth A. Gross, MD, FACG
Associate Professor of Medicine, NYU School of Medicine; Chief of Gastroenterology, Tisch Hospital
NYU Langone Health
New York, NY
Presenting Author(s)
Seth A. Gross, MD1, Terilyn R. Scott-Winful, MD2, Jean Wang, MD, PhD3
1NYU Langone Health, New York, NY; 2Baylor, Scott & White Digestive Diseases, Plano, TX; 3Washington University School of Medicine in St. Louis, St. Louis, MO
Introduction: Looping during colonoscopy increases scope forces and torquing which are causes of ergonomic injury among endoscopists. In addition, manual abdominal pressure and patient repositioning, used to address looping in 52% and 34% of colonoscopies, respectively, are known causes of musculoskeletal injuries among endoscopy staff. ColoWrap (ColoWrap, LLC, Durham, NC) is an anti-looping abdominal compression device applied during colonoscopy to decrease looping and limit the need for manual pressure and patient repositioning. We aimed to determine extent to which ColoWrap reduces ergonomic hazards associated with colonoscopy by performing a chart review and obtaining physician and staff feedback following use of the device.
Methods: This retrospective, multi-center, observational chart review included patients that underwent colonoscopy with the ColoWrap device between September 25, 2016, and June 15, 2022. Demographics and procedural information were abstracted from patient records. Physician and staff experiences were captured using a survey instrument.
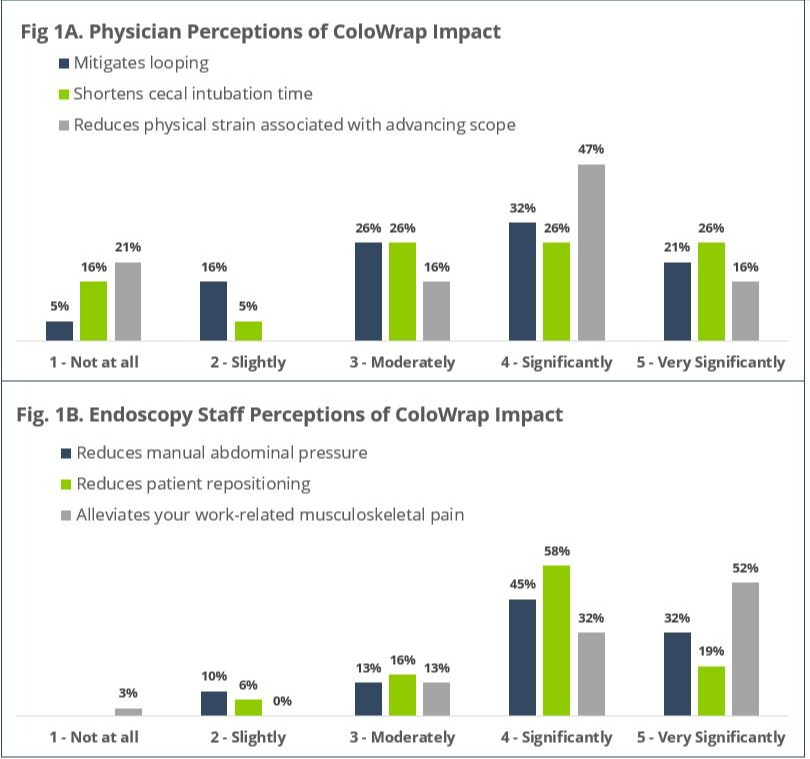
Results: 849 procedures were included in the review. The population was majority male (53%), over 60 (mean age: 60.8 ± 11.6), and obese (mean BMI: 33.6 ± 7.2). 49 patients (5.7%) had an abdominal hernia, 139 (16.3%) had at least one prior abdominal surgery, and 52 (6.1%) had a history of difficult or incomplete colonoscopy. Cecal intubation was achieved in 841 cases (99.1%). Mean cecal intubation time was 6.8 ± 6.2 (min). Manual pressure was used in 109 cases (12.8%); significant manual pressure ( > 3 min) was needed in only 21 procedures (2.5%). Patient repositioning was used in 48 cases (5.6%). No significant adverse events were reported. 84% of physicians indicated that ColoWrap use mitigated looping, shortened cecal intubation time, and reduced physical strain associated with advancing the scope. 90% of endoscopy staff reported reduced manual pressure and patient repositioning, and alleviation of musculoskeletal pain.
Discussion: ColoWrap is safe and significantly reduces manual pressure and patient repositioning during colonoscopy relative to published rates. Physicians using ColoWrap experience less looping and physical strain and endoscopy staff suffer less musculoskeletal pain. The device is a viable tool among solutions to improve the safety and efficiency of colonoscopy. Further studies to identify circumstances in which ColoWrap use offers the greatest benefit to patients, physicians, and staff are warranted.
Disclosures:
Seth A. Gross, MD1, Terilyn R. Scott-Winful, MD2, Jean Wang, MD, PhD3. C0291 - ColoWrap Real-World Evidence: Colonoscopy Compression Device Mitigates Ergonomic Hazards for Endoscopists and Staff, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1NYU Langone Health, New York, NY; 2Baylor, Scott & White Digestive Diseases, Plano, TX; 3Washington University School of Medicine in St. Louis, St. Louis, MO
Introduction: Looping during colonoscopy increases scope forces and torquing which are causes of ergonomic injury among endoscopists. In addition, manual abdominal pressure and patient repositioning, used to address looping in 52% and 34% of colonoscopies, respectively, are known causes of musculoskeletal injuries among endoscopy staff. ColoWrap (ColoWrap, LLC, Durham, NC) is an anti-looping abdominal compression device applied during colonoscopy to decrease looping and limit the need for manual pressure and patient repositioning. We aimed to determine extent to which ColoWrap reduces ergonomic hazards associated with colonoscopy by performing a chart review and obtaining physician and staff feedback following use of the device.
Methods: This retrospective, multi-center, observational chart review included patients that underwent colonoscopy with the ColoWrap device between September 25, 2016, and June 15, 2022. Demographics and procedural information were abstracted from patient records. Physician and staff experiences were captured using a survey instrument.
Results: 849 procedures were included in the review. The population was majority male (53%), over 60 (mean age: 60.8 ± 11.6), and obese (mean BMI: 33.6 ± 7.2). 49 patients (5.7%) had an abdominal hernia, 139 (16.3%) had at least one prior abdominal surgery, and 52 (6.1%) had a history of difficult or incomplete colonoscopy. Cecal intubation was achieved in 841 cases (99.1%). Mean cecal intubation time was 6.8 ± 6.2 (min). Manual pressure was used in 109 cases (12.8%); significant manual pressure ( > 3 min) was needed in only 21 procedures (2.5%). Patient repositioning was used in 48 cases (5.6%). No significant adverse events were reported. 84% of physicians indicated that ColoWrap use mitigated looping, shortened cecal intubation time, and reduced physical strain associated with advancing the scope. 90% of endoscopy staff reported reduced manual pressure and patient repositioning, and alleviation of musculoskeletal pain.
Discussion: ColoWrap is safe and significantly reduces manual pressure and patient repositioning during colonoscopy relative to published rates. Physicians using ColoWrap experience less looping and physical strain and endoscopy staff suffer less musculoskeletal pain. The device is a viable tool among solutions to improve the safety and efficiency of colonoscopy. Further studies to identify circumstances in which ColoWrap use offers the greatest benefit to patients, physicians, and staff are warranted.

Figure: Endoscopist and Endoscopy Staff Experience with ColoWrap Device
Disclosures:
Seth Gross: ColoWrap, LLC – Advisory Committee/Board Member. Olympus – Consultant.
Terilyn Scott-Winful indicated no relevant financial relationships.
Jean Wang indicated no relevant financial relationships.
Seth A. Gross, MD1, Terilyn R. Scott-Winful, MD2, Jean Wang, MD, PhD3. C0291 - ColoWrap Real-World Evidence: Colonoscopy Compression Device Mitigates Ergonomic Hazards for Endoscopists and Staff, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
