Back

Poster Session A - Sunday Afternoon
Category: Liver
A0541 - Early Use of Tocilizumab as an Effective Steroid-Sparing Strategy for the Treatment of Immune Checkpoint Inhibitor-Mediated Cholangiopathy: Building Foundations for Personalized Management
Sunday, October 23, 2022
5:00 PM – 7:00 PM ET
Location: Crown Ballroom

Has Audio

Hao Chi Zhang, MD
UT MD Anderson Cancer Center
Houston, TX
Presenting Author(s)
Hao Chi Zhang, MD1, Ethan D. Miller, MD2, Lan Wang, MD3
1UT MD Anderson Cancer Center, Houston, TX; 2University of Texas MD Anderson Cancer Center, Houston, TX; 3MD Anderson Cancer Center, Houston, TX
Introduction: Immune-mediated cholangiopathy (IMCp) is an increasingly recognized complication of immune checkpoint inhibitor therapy, often associated with exposure to anti-PD-1/L1 agents. Both intra- and extrahepatic manifestations can occur. Potential sequelae include biliary strictures and acute cholangitis. The management of IMCp remains undefined across multiple society guidelines, but published cases have offered insight into formulating effective treatment strategies. We present a complex case of a patient with IMCp successfully treated with budesonide and early tocilizumab, an IL-6 receptor antagonist.
Case Description/Methods: A 70-year-old man with a history of urothelial carcinoma (treated with neoadjuvant carboplatin/gemcitabine, followed by 3 cycles of an anti-PD-1 agent, pembrolizumab), pre-diabetes, and no underlying liver disease, presented with abnormal liver enzymes. ERCP revealed some biliary sludge but no biliary stricture. Endoscopic ultrasound 3 months later was normal. ALT normalized, but alkaline phosphatase (ALP) remained elevated; both then increased to CTCAE grade 2. R factor was 0.5. Elective cholecystectomy was attempted but aborted: He had a firm, contracted gallbladder with dilated extrahepatic biliary ducts, and the surgeon could not demarcate borders of the cystic and common bile ducts. MRCP showed intrahepatic biliary ductal dilation, ductal thickening and enhancement, and CBD dilation with a new stricture. Liver biopsy showed cholestatic hepatitis with portal and lobular inflammation and hepatocyte necrosis. A diagnosis of immune-mediated cholangiohepatitis with IMCp was made. Oral budesonide/azathioprine/ursodiol were prescribed. Five days later, subcutaneous tocilizumab 162 mg was administered without complication. After completion of budesonide/azathioprine, ursodiol was continued long-term for persistent elevation of ALP. Serial ERCPs for plastic biliary stent placement were performed for biliary sludge and for an unresolved CBD stricture. ALP level improved to < 180 U/L about 7-8 months after tocilizumab.
Discussion: Cases of IMCp could be potentially overlooked because ALP is not featured in the society guidelines' assessments for liver toxicity. Although steroids can be used to initially treat IMCp, aggressive escalation to alternative treatments such as tocilizumab is important to mitigate progression to biliary sequelae that might arise. As use of anti-PD-1/L1 agents expand, IMCp may become increasingly common, and prompt treatment is of paramount importance.

Disclosures:
Hao Chi Zhang, MD1, Ethan D. Miller, MD2, Lan Wang, MD3. A0541 - Early Use of Tocilizumab as an Effective Steroid-Sparing Strategy for the Treatment of Immune Checkpoint Inhibitor-Mediated Cholangiopathy: Building Foundations for Personalized Management, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1UT MD Anderson Cancer Center, Houston, TX; 2University of Texas MD Anderson Cancer Center, Houston, TX; 3MD Anderson Cancer Center, Houston, TX
Introduction: Immune-mediated cholangiopathy (IMCp) is an increasingly recognized complication of immune checkpoint inhibitor therapy, often associated with exposure to anti-PD-1/L1 agents. Both intra- and extrahepatic manifestations can occur. Potential sequelae include biliary strictures and acute cholangitis. The management of IMCp remains undefined across multiple society guidelines, but published cases have offered insight into formulating effective treatment strategies. We present a complex case of a patient with IMCp successfully treated with budesonide and early tocilizumab, an IL-6 receptor antagonist.
Case Description/Methods: A 70-year-old man with a history of urothelial carcinoma (treated with neoadjuvant carboplatin/gemcitabine, followed by 3 cycles of an anti-PD-1 agent, pembrolizumab), pre-diabetes, and no underlying liver disease, presented with abnormal liver enzymes. ERCP revealed some biliary sludge but no biliary stricture. Endoscopic ultrasound 3 months later was normal. ALT normalized, but alkaline phosphatase (ALP) remained elevated; both then increased to CTCAE grade 2. R factor was 0.5. Elective cholecystectomy was attempted but aborted: He had a firm, contracted gallbladder with dilated extrahepatic biliary ducts, and the surgeon could not demarcate borders of the cystic and common bile ducts. MRCP showed intrahepatic biliary ductal dilation, ductal thickening and enhancement, and CBD dilation with a new stricture. Liver biopsy showed cholestatic hepatitis with portal and lobular inflammation and hepatocyte necrosis. A diagnosis of immune-mediated cholangiohepatitis with IMCp was made. Oral budesonide/azathioprine/ursodiol were prescribed. Five days later, subcutaneous tocilizumab 162 mg was administered without complication. After completion of budesonide/azathioprine, ursodiol was continued long-term for persistent elevation of ALP. Serial ERCPs for plastic biliary stent placement were performed for biliary sludge and for an unresolved CBD stricture. ALP level improved to < 180 U/L about 7-8 months after tocilizumab.
Discussion: Cases of IMCp could be potentially overlooked because ALP is not featured in the society guidelines' assessments for liver toxicity. Although steroids can be used to initially treat IMCp, aggressive escalation to alternative treatments such as tocilizumab is important to mitigate progression to biliary sequelae that might arise. As use of anti-PD-1/L1 agents expand, IMCp may become increasingly common, and prompt treatment is of paramount importance.

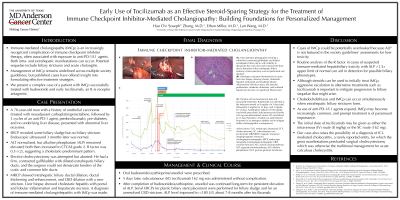
Figure: (A): Intra-operative photographs revealing a white/firm contracted gallbladder and dilated extrahepatic biliary ducts, with inability to demarcate the cystic duct and common bile duct; due to distortion of the extrahepatic biliary anatomy, cholecystectomy was not performed and aborted. (B): Histologic evaluation (hematoxylin & eosin) from liver biopsy, showing chronic cholestatic hepatitis with portal and moderate lobular inflammation, bile duct injury, bile ductular proliferation, moderate cholestasis, and scattered hepatocyte necrosis; no significant fibrosis seen. (C): Timeline of liver biochemical tests and associated treatments. Budesonide was selected as the induction steroid, at 9 mg/day, for 5 days total, followed by 6 mg/day for 14 days, and 3 mg/day for 14 days. Azathioprine adjunct of 100 mg/day was briefly prescribed. Subcutaneous tocilizumab 162 mg was administered; serum ALT normalized at 31 days thereafter. Ursodiol was administered long-term in an attempt to address and to mitigate further cholangiopathic consequences. Abbreviations: EUS, endoscopic ultrasound; CCY, cholecystectomy; SC, subcutaneous; toci, tocilizumab; MRI/MRCP, magnetic resonance imaging/magnetic resonance cholangiopancreatography; ERCP, endoscopic retrograde cholangiopancreatography; CBD, common bile duct; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; GGT, gamma-glutamyl transferase.
Disclosures:
Hao Chi Zhang indicated no relevant financial relationships.
Ethan Miller indicated no relevant financial relationships.
Lan Wang indicated no relevant financial relationships.
Hao Chi Zhang, MD1, Ethan D. Miller, MD2, Lan Wang, MD3. A0541 - Early Use of Tocilizumab as an Effective Steroid-Sparing Strategy for the Treatment of Immune Checkpoint Inhibitor-Mediated Cholangiopathy: Building Foundations for Personalized Management, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.

