Back

Poster Session A - Sunday Afternoon
Category: Colon
A0104 - Patient-Derived Enteroids and Colonoids for Measuring Enteroendocrine Function in Response to Luminal Contents: A Proof-of-Concept Study in Parkinson’s Disease
Sunday, October 23, 2022
5:00 PM – 7:00 PM ET
Location: Crown Ballroom

Has Audio

Richard A. Manfready, MD, AM
Rush University Medical Center
Chicago, IL
Presenting Author(s)
Richard A. Manfready, MD, AM1, Maliha W. Shaikh, MS1, Lijuan Zhang, MD1, Kristi M. Lawrence, 1, Robin M. Voigt, PhD1, Christopher B. Forsyth, PhD1, Christopher G. Goetz, MD1, Ali Keshavarzian, MD2
1Rush University Medical Center, Chicago, IL; 2Rush Center for Integrated Microbiome and Chronobiology Research, Chicago, IL
Introduction: In vitro studies of the gut-brain axis will require precise models of enteroendocrine function that replicate both patient-specific phenotype and intestinal milieu. Here we establish one such model for measuring the effects of stool and short-chain fatty acids (SCFA) on L-cell activity in Parkinson’s disease (PD). We have adapted a patient-derived organoid system which replicates intestinal epithelial function, immune responsiveness, and barrier integrity to respond directly to luminal contents including stool. Given the emerging importance of the enteroendocrine system in mediating microbiota-derived gut-brain signals in PD and other neurologic diseases, our system provides a needed platform for querying L-cells from a single stem-cell niche.
Methods: Ileal and sigmoid colon biopsies were taken from PD patients and matched healthy volunteers between the ages of 40 and 80 years. 3D spherical organoids were generated from crypts isolated by EDTA, embedded in an extracellular matrix protein scaffold, and treated with growth factors to expand Lgr5+ stem cells through serial passages. Organoid polarity was inverted such that the apical surface faced outward. Enteroids and colonoids were then cocultured with stool supernatant or SCFA mixtures that resembled PD or control intestinal milieu. Glucagon-like peptide 1 (GLP-1) secreted by L-cells into the spheres was measured by ELISA after lysis and normalized to DNA content.
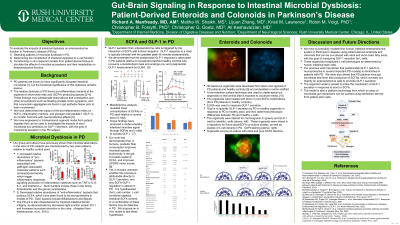
Results: 3D epithelial organoids were successfully generated from ileal and colonic crypts from 7 PD and 7 control patients. L-cell presence was verified by immunohistochemistry for GLP-1 and chromogranin A. Spherical organoids were successfully inverted before coculture. GLP-1 secretion by control organoids was diminished in the presence of PD stool or SCFA relative to control milieu, and secretion by PD organoids was further diminished in either coculture condition.
Discussion: This proof-of-concept study establishes patient-derived organoids as a platform technology for measuring enteroendocrine responses to luminal contents, including stool and SCFA. Here, we use this technique to demonstrate that PD and healthy L-cells respond differently to SCFA in the stool. However, our novel system may be adapted to study a vast array of patient-specific enteroendocrine responses to stool microbiota, metabolites, and drugs.
Disclosures:
Richard A. Manfready, MD, AM1, Maliha W. Shaikh, MS1, Lijuan Zhang, MD1, Kristi M. Lawrence, 1, Robin M. Voigt, PhD1, Christopher B. Forsyth, PhD1, Christopher G. Goetz, MD1, Ali Keshavarzian, MD2. A0104 - Patient-Derived Enteroids and Colonoids for Measuring Enteroendocrine Function in Response to Luminal Contents: A Proof-of-Concept Study in Parkinson’s Disease, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1Rush University Medical Center, Chicago, IL; 2Rush Center for Integrated Microbiome and Chronobiology Research, Chicago, IL
Introduction: In vitro studies of the gut-brain axis will require precise models of enteroendocrine function that replicate both patient-specific phenotype and intestinal milieu. Here we establish one such model for measuring the effects of stool and short-chain fatty acids (SCFA) on L-cell activity in Parkinson’s disease (PD). We have adapted a patient-derived organoid system which replicates intestinal epithelial function, immune responsiveness, and barrier integrity to respond directly to luminal contents including stool. Given the emerging importance of the enteroendocrine system in mediating microbiota-derived gut-brain signals in PD and other neurologic diseases, our system provides a needed platform for querying L-cells from a single stem-cell niche.
Methods: Ileal and sigmoid colon biopsies were taken from PD patients and matched healthy volunteers between the ages of 40 and 80 years. 3D spherical organoids were generated from crypts isolated by EDTA, embedded in an extracellular matrix protein scaffold, and treated with growth factors to expand Lgr5+ stem cells through serial passages. Organoid polarity was inverted such that the apical surface faced outward. Enteroids and colonoids were then cocultured with stool supernatant or SCFA mixtures that resembled PD or control intestinal milieu. Glucagon-like peptide 1 (GLP-1) secreted by L-cells into the spheres was measured by ELISA after lysis and normalized to DNA content.
Results: 3D epithelial organoids were successfully generated from ileal and colonic crypts from 7 PD and 7 control patients. L-cell presence was verified by immunohistochemistry for GLP-1 and chromogranin A. Spherical organoids were successfully inverted before coculture. GLP-1 secretion by control organoids was diminished in the presence of PD stool or SCFA relative to control milieu, and secretion by PD organoids was further diminished in either coculture condition.
Discussion: This proof-of-concept study establishes patient-derived organoids as a platform technology for measuring enteroendocrine responses to luminal contents, including stool and SCFA. Here, we use this technique to demonstrate that PD and healthy L-cells respond differently to SCFA in the stool. However, our novel system may be adapted to study a vast array of patient-specific enteroendocrine responses to stool microbiota, metabolites, and drugs.
Disclosures:
Richard Manfready indicated no relevant financial relationships.
Maliha Shaikh indicated no relevant financial relationships.
Lijuan Zhang indicated no relevant financial relationships.
Kristi Lawrence indicated no relevant financial relationships.
Robin Voigt indicated no relevant financial relationships.
Christopher Forsyth indicated no relevant financial relationships.
Christopher Goetz indicated no relevant financial relationships.
Ali Keshavarzian indicated no relevant financial relationships.
Richard A. Manfready, MD, AM1, Maliha W. Shaikh, MS1, Lijuan Zhang, MD1, Kristi M. Lawrence, 1, Robin M. Voigt, PhD1, Christopher B. Forsyth, PhD1, Christopher G. Goetz, MD1, Ali Keshavarzian, MD2. A0104 - Patient-Derived Enteroids and Colonoids for Measuring Enteroendocrine Function in Response to Luminal Contents: A Proof-of-Concept Study in Parkinson’s Disease, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.

