VERRICA Pharmaceuticals
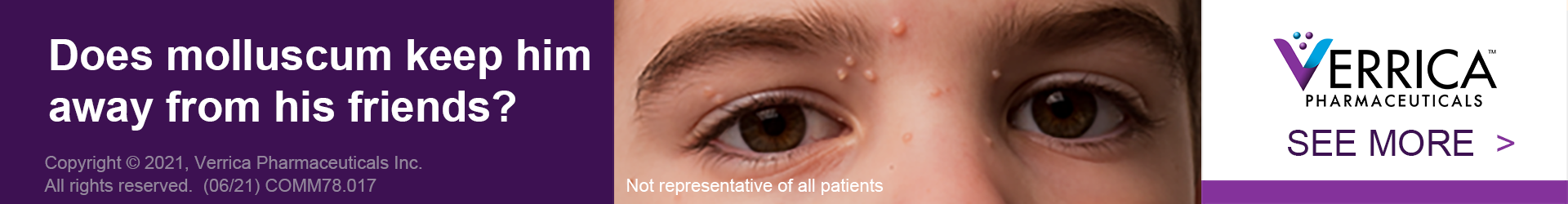
Verrica Pharmaceuticals Inc. is a dermatology therapeutics company developing medications for skin diseases requiring medical interventions. We are focused on changing the narrative around skin diseases and making treatment a more efficient process for both physicians and patients. VP-102, our lead product candidate, is a drug-device combination topical therapy that has the potential to be the first product approved by the FDA to treat molluscum contagiosum (molluscum). If approved, VP-102 will be marketed in the United States under the conditionally accepted brand name, YCANTH™. Results suggest that YCANTH™, if approved, could potentially improve both clinical practice and patient outcomes by providing molluscum patients with a new treatment option.
VERRICA Pharmaceuticals
Verrica Pharmaceuticals, Inc.
44 W Gay St.
Ste. 400
WEST CHESTER, PA 19380
