Category: COVID-19 Treatment
Poster Session: COVID-19 Treatment
553 - Critically Ill patients Receiving Tocilizumab Compared With Those Not Receiving Tocilizumab for Treatment of COVID-19
- ML
Michael Leonard
Professor of Medicine
Carolinas Medical Center Atrium Health
Charlotte, North CarolinaDisclosure: I do not have any relevant financial / non-financial relationships with any proprietary interests.
- aA
anthony Asher
Medical Student
UNC School of Medicine
Charlotte, North CarolinaDisclosure: I do not have any relevant financial / non-financial relationships with any proprietary interests.
- BK
Banks Kooken
Medical Student
UNC School of Medicine
Charlotte, North CarolinaDisclosure: I do not have any relevant financial / non-financial relationships with any proprietary interests.
- ED
Erin Donahue
Dr.
Levine Cancer Center
charlotte, North CarolinaDisclosure: I do not have any relevant financial / non-financial relationships with any proprietary interests.
- jS
james Symanowski
Dr
Levine cancer Institute
charlotte, North CarolinaDisclosure: I do not have any relevant financial / non-financial relationships with any proprietary interests.
- DR
Danya Roshdy
Clinical Pharmacy Specialist, Infectious Diseases
Atrium Health
Charlotte, North CarolinaDisclosure: I do not have any relevant financial / non-financial relationships with any proprietary interests.
- jo
jennifer onsrud
PharmD
Atrium Health
charlotte, North CarolinaDisclosure: I do not have any relevant financial / non-financial relationships with any proprietary interests.
- SU
Saad Umani
Dr
Levine Cancer Institute
charlotte, North CarolinaDisclosure: I do not have any relevant financial / non-financial relationships with any proprietary interests.
- eC
edward Copelan
Dr
Levine Cancer Institute
charlotte, North CarolinaDisclosure: I do not have any relevant financial / non-financial relationships with any proprietary interests.
- zs
zainab shahid
Dr
Levine cancer Institute
charlotte, North CarolinaDisclosure: I do not have any relevant financial / non-financial relationships with any proprietary interests.
Presenting Author(s)
Co-Author(s)
Background:
Background: :
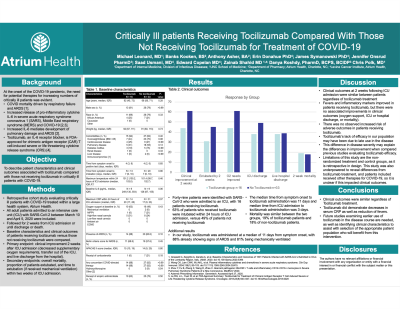
Immune modulation in patients with clinical features suggestive of a cytokine release syndrome (CRS) has become a pharmacologic target for potential treatment of COVID-19 and prevention of ARDS. Tocilizumab is an IL-6 receptor blocker FDA-approved for chimeric antigen receptor (CAR) T cell-induced severe or life-threatening CRS. The objective of this study was to describe clinical outcomes associated with tocilizumab compared with those not receiving tocilizumab in critically ill patients with severe COVID-19.
Methods:
Methods:
Retrospective case series of 49 adult patients admitted to an intensive care unit with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Patients receiving tocilizumab were compared with those not receiving tocilizumab. The primary outcome was clinical improvement (decrease in supplemental oxygen requirement, discharge from ICU, or live discharge from hospital). Secondary endpoints included mortality and frequency of extubation. All comparative endpoints were assessed at 2 weeks after ICU admission.
Results:
Results:
49 patients were identified with SARS-CoV-2 who were admitted to an ICU, 16 received tocilizumab. Baseline characteristics were similar; most were African American males with comorbidities such as obesity, cardiovascular disease, and diabetes. The time from symptom onset to positive test and subsequent intubation were similar (4 and 7 days, respectively). 75% received one dose (all received 8 mg/kg). The median time from symptom onset to tocilizumab administration was 11 days.
In patients receiving tocilizumab compared with those not receiving tocilizumab, there were similar rates of clinical improvement (44% versus 61%, p=0.27), extubation (31% versus 45%, p=0.60), and mortality (18% versus 19%, p >0.99, respectively). 81% of the tocilizumab group had resolution of fever and 75% had improvement in C-reactive protein levels.
Conclusion:
Conclusion:
In this study of patients with progressed disease, outcomes were similar regardless of receipt of tocilizumab. Randomized controlled trials are needed to assess the impact of earlier administration and identify clinical characteristics to assist with selection of appropriate patients who may benefit from tocilizumab.

