Category: Hepatitis
Poster Session: Hepatitis
1074 - Understanding Screening Practices for Hepatitis B Prior to Starting Biologics at an Academic Medical Center.

Tulip A. Jhaveri
Infectious Disease Fellow
Tufts Medical Center, Boston, MA 02111
Boston, MassachusettsDisclosure: I do not have any relevant financial / non-financial relationships with any proprietary interests.
- JH
James B. Higgs
Infectious Disease Attending
Kent Hospital, Warwick, RI 02886
Warwick, Rhode IslandDisclosure: I do not have any relevant financial / non-financial relationships with any proprietary interests.
- MH
Mary J. Hopkins
Associate Program Director of the Infectious Diseases Fellowship Program
Tufts Medical Center, Boston, MA 02111
Boston, MassachusettsDisclosure: I do not have any relevant financial / non-financial relationships with any proprietary interests.
Presenting Author(s)
Co-Author(s)
Background:
It is estimated that 0.3% of the US population has chronic hepatitis B (HBV) infection, most of whom are asymptomatic. When a patient receives a biologic medication, chronic HBV can reactivate with mortality rates as high as 40%. We aim to understand HBV screening practices prior to starting biologics at a single tertiary academic medical center.
Methods:
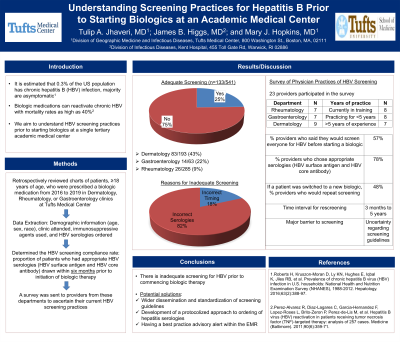
We retrospectively reviewed over 500 patient charts. These patients aged ≥ 18 years were prescribed a biologic medication at one of the three clinics (Dermatology, Rheumatology, or Gastroenterology) at Tufts Medical Center from January 2016 to April 2019. To determine the rate of HBV screening compliance, we reported the proportion of patients who had appropriate HBV serologies (HBV surface antigen and HBV core antibody) drawn prior to initiation of the biologic therapy. A survey was sent to providers from these departments to understand their current practices of HBV screening.
Results: 133 of 541 patients (25%) had been appropriately screened for HBV within six months prior to starting biologic therapy. 207 of 541 (38%) had been screened with appropriate serologies within ten years prior to starting a biologic. 23 providers participated in the survey, 7 each from the department of Rheumatology and Gastroenterology, and 9 from Dermatology. One-third of the providers were currently in training, another third were practicing for < 5 years, and the remainder had > 5 years of experience. 57% of the providers said they would screen everyone for HBV before starting a biologic. 78% of them chose the appropriate serologies. The time interval for rescreening was evenly spread amongst different providers, ranging from 3 months to 5 years. If a patient was switched to a new biologic, 48% of physicians would repeat screening only if the patient was determined to be at risk of reactivation or new acquisition of HBV. The major barrier to screening was uncertainty regarding who to screen and which tests to order.
Conclusion: This data reveals that there is inadequate screening for HBV prior to biologic therapy. The survey highlighted areas for quality improvement, including the need for wider dissemination of screening guidelines and development of a protocolized approach to ordering the correct tests.

