Category: COVID-19 Treatment
Poster Session: COVID-19 Treatment
564 - Tocilizumab Induces Rapid, Sustained Improvement of Inflammatory Markers in COVID-19.

Robert Colgrove
Interim Chief of Infectious Diseases, Assistant Professor of Medicine
Mount Auburn Hospital, Harvard Medical School
Cambridge, MassachusettsDisclosure: I do not have any relevant financial / non-financial relationships with any proprietary interests.
- SM
Scott Morin
Resident in Internal Medicine, Mount Auburn Hospital
Department of Medicine, Mount Auburn Hospital
Cambridge, MassachusettsDisclosure: I do not have any relevant financial / non-financial relationships with any proprietary interests.
- CJ
Chinmay Jani
Resident in Internal Medicine, Mount Auburn Hospital
Department of Medicine, Mount Auburn Hospital
Cambridge, MassachusettsDisclosure: I do not have any relevant financial / non-financial relationships with any proprietary interests.
- AR
Arashdeep Rupal
Resident in Internal Medicine, Mount Auburn Hospital
Department of Medicine, Mount Auburn Hospital
Cambridge, MassachusettsDisclosure: I do not have any relevant financial / non-financial relationships with any proprietary interests.
- DB
Daniel L. Bourque
Instructor
Mount Auburn Hospital, Harvard Medical School
Cambridge, MassachusettsDisclosure: I do not have any relevant financial / non-financial relationships with any proprietary interests.
Presenting Author(s)
Co-Author(s)
Background:
Frequent observation of increasing fever and rising inflammatory markers late after onset of COVID-19 suggests Cytokine Release Syndrome (CRS, "Cytokine Storm") may contribute to pathophysiology. Tocilizumab (TCZ), a monoclonal antibody targeting the receptor for the pro-inflammatory cytokine, IL-6, is effective in suppressing pathological inflammation in several rheumatological diseases. After administering TCZ to COVID-19 patients with suspected CRS, we observed a sharp fall in inflammatory indices. We analyzed this effect using results from the first 19 COVID-19 patients receiving TCZ at our hospital.
Methods:
Data for all patients with confirmed COVID-19 who received TCZ at our center, a 200 bed community hospital in New England, were extracted from the Electronic Medical Record, including demographics, body temperature, C-Reactive Protein (CRP), IL-6 levels, clinical severity on the Ordinal Scale for Clinical Improvement (OSCI), and clinical outcome (recovery/discharge home, partial recovery/discharge rehab, death). Results were tabulated and statistical significance of changes in indices pre- and post- TCZ assessed by Wilcoxon Signed-Rank Test.
Results:
19 patients received TCZ: 16 got 400mg x1, 2 got 400 mg x2, 1 got 660 mg x1. Median age was 64 years (range: 44-94), 68% male. Mean interval from symptom onset to receiving TCZ was 11.5 days. Mean IL-6 was 145 pg/mL. Demographics, OSCI scores, and discharge status are shown in Table 1. Average daily peak temperatures (Tmax) pre- and post- TCZ were 100.7 and 98.9°F, p< 0.001. Mean CRP pre- and post- were 234 and 84.6 mg/L, p=0.001 (Fig.1). Decrease in Tmax and CRP was rapid and sustained (Fig. 2, 1st 8 patients shown for clarity.). 58% had improved clinical improvement by OSCI by day 7, 68% by day 14. 7 of 19 of patients were discharged home, 6 to rehab or acute care facility, and 6 died. Table 1: Patient Demographics, Clinical Severity Score, and Discharge Status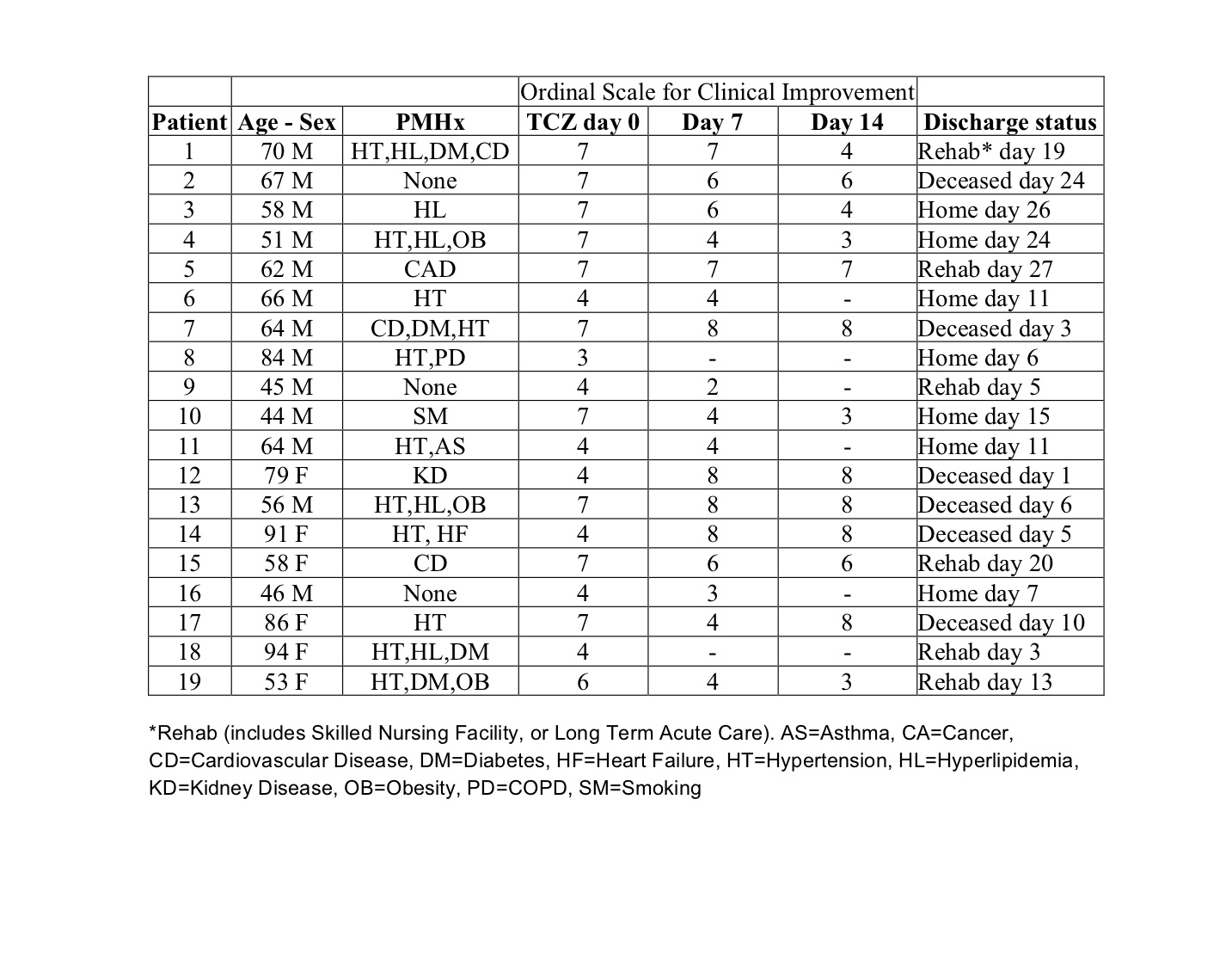 Figure 1: CRP and Tmax pre- and post- Tocilizumab
Figure 1: CRP and Tmax pre- and post- Tocilizumab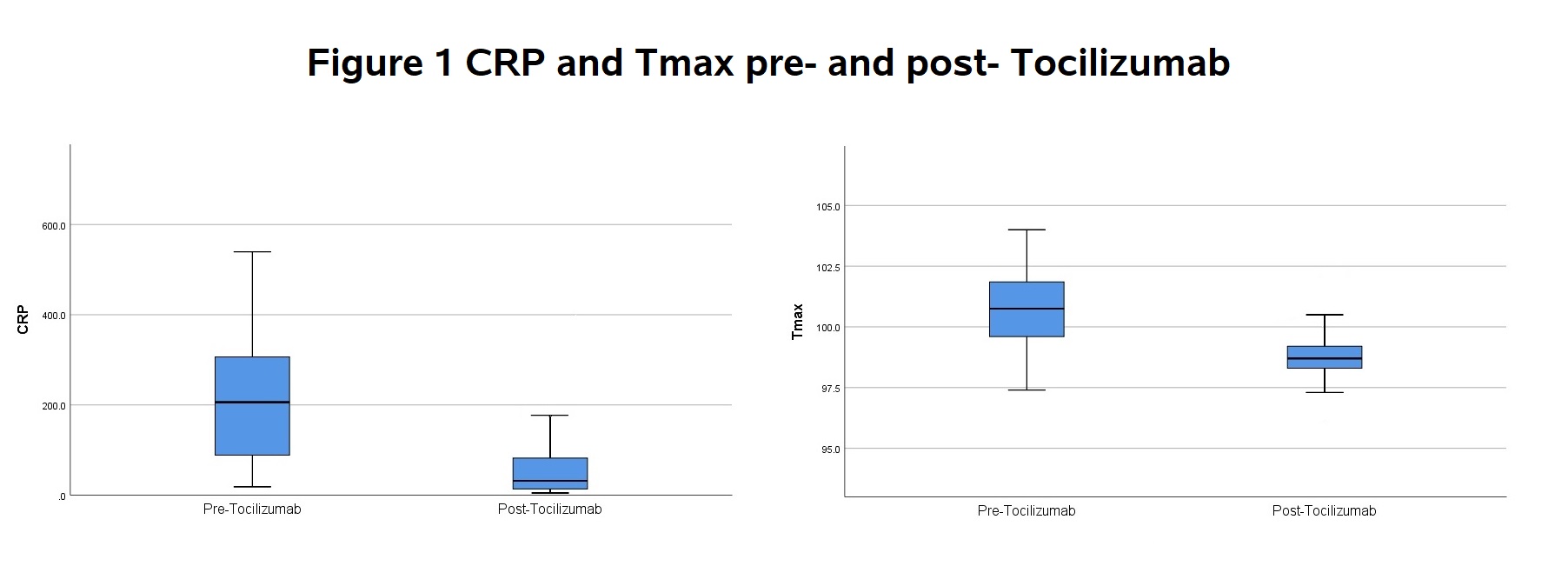 Figure 2: Time Course of Temperature and CRP after Tocilizumab
Figure 2: Time Course of Temperature and CRP after Tocilizumab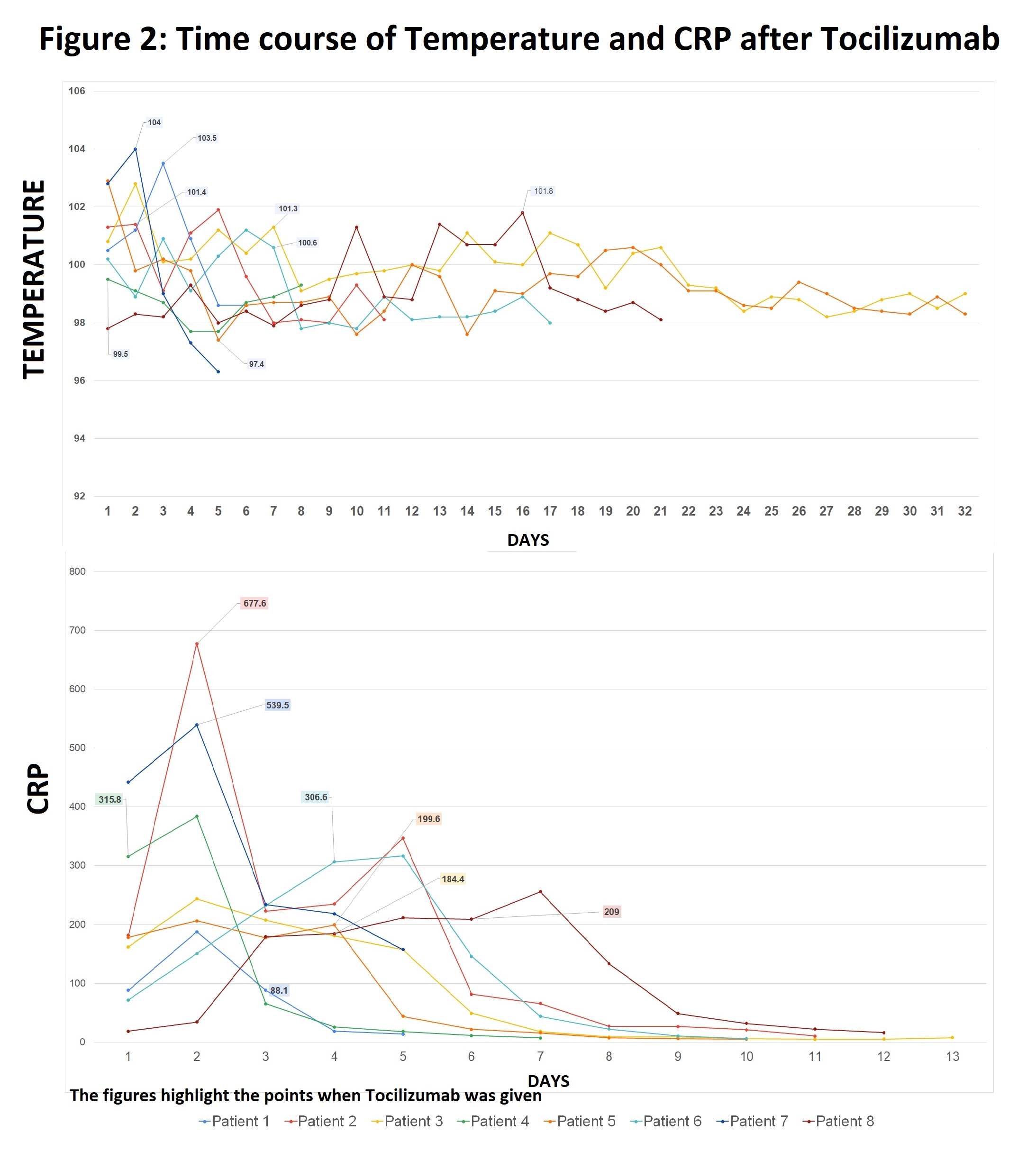
Conclusion:
In this cohort of patients with moderate-to-severe COVID-19 and evidence of Cytokine Release Syndrome, tocilizumab was associated with rapid resolution of fever and marked decline in CRP. Most patients showed improvement in clinical severity scores and no adverse reactions were noted. Tocilizumab may be useful in control of pathological inflammation in COVID-19. Controlled trials will be needed to assess overall clinical benefit.

